Trusted by the most innovative organizations
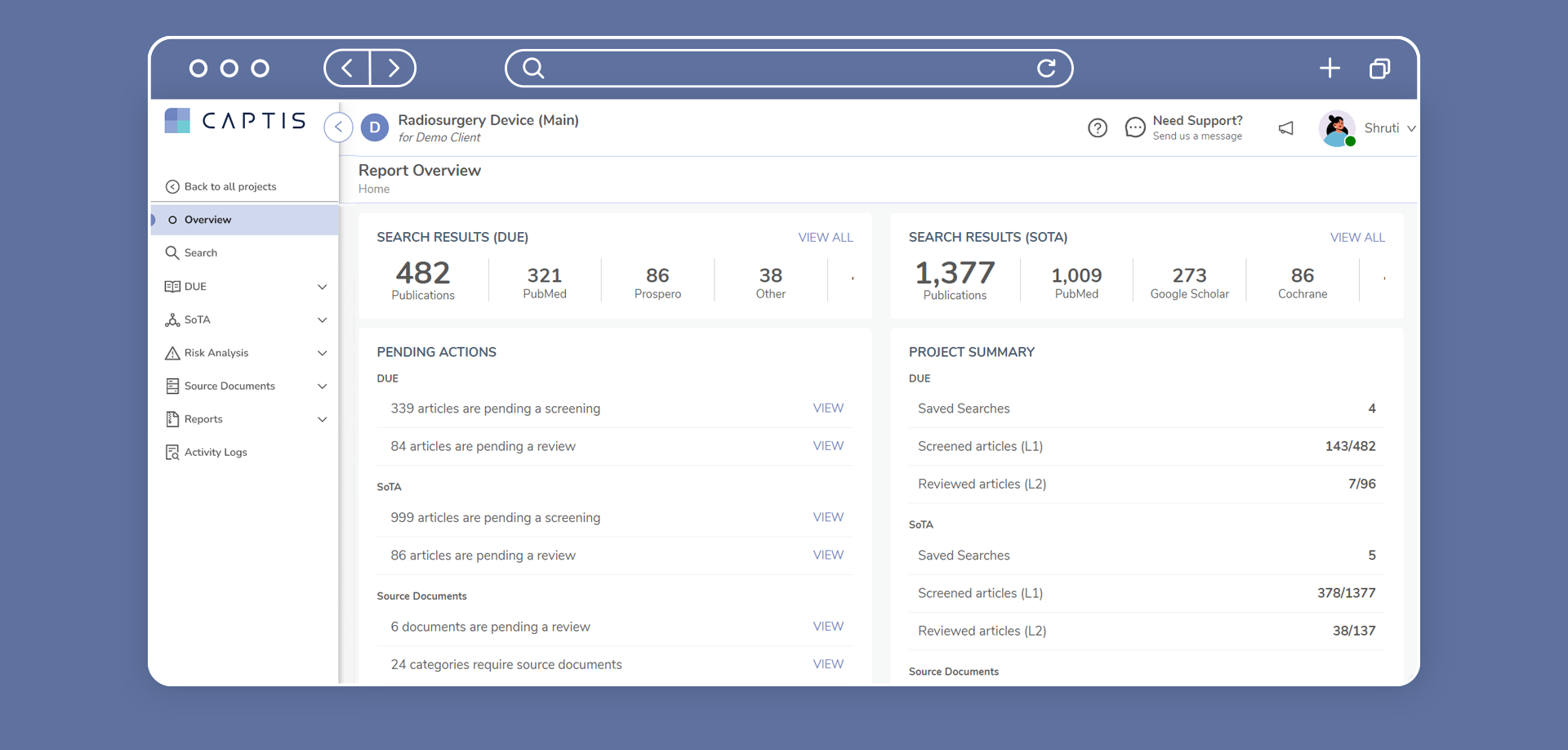
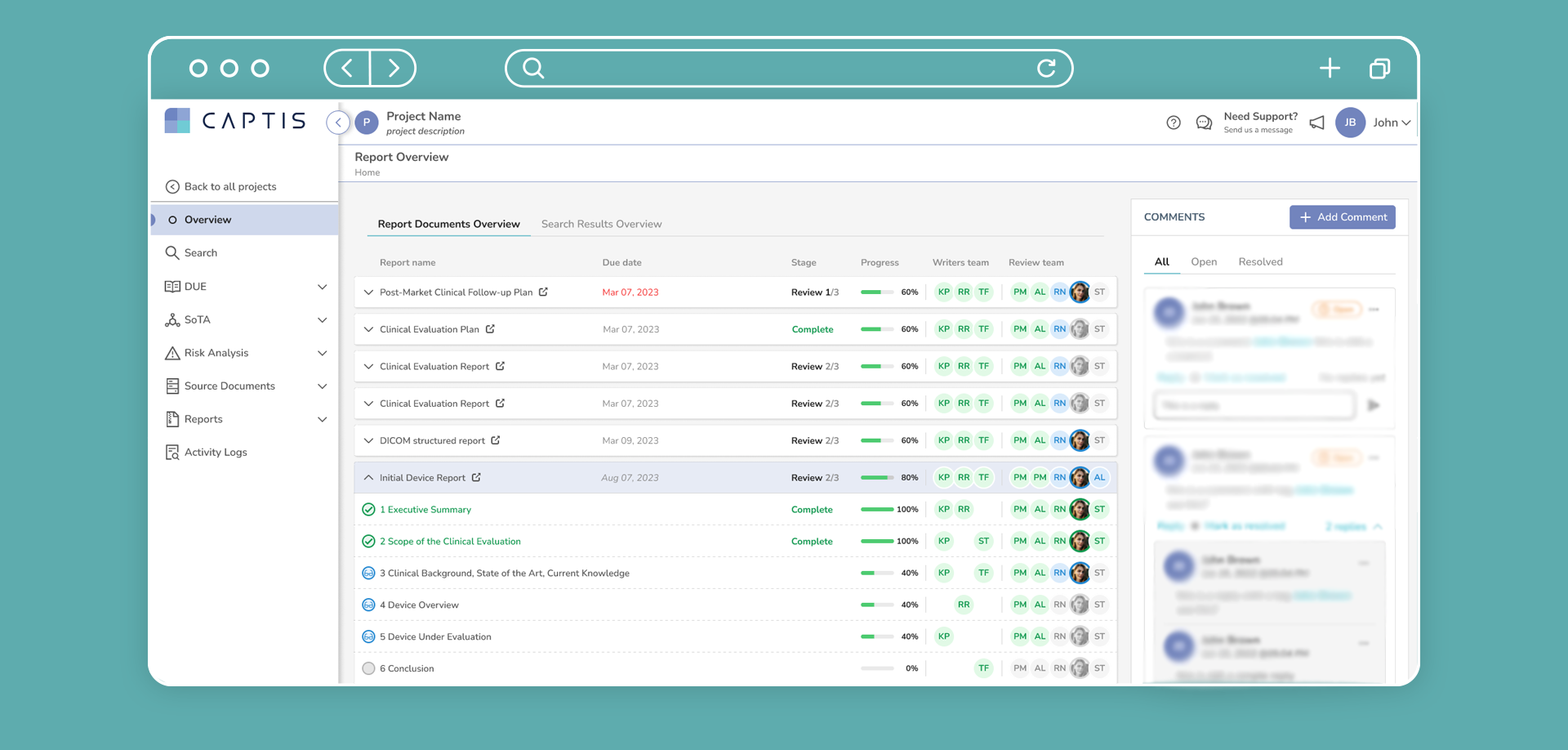
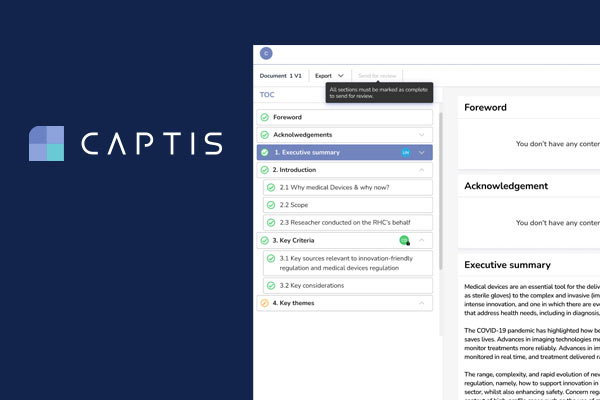
CAPTIS™ streamlines all MDR & IVDR document creation. The system can be used to create multiple types of reports more efficiently, including CERs, PERs, SSCPs, and PSURs.
The CAPTIS solution allows your team to write PMS documentation in much less time and avoid constant revisions. It not only improves efficiency and quality of medical writing teams, but also allows your project manager to quickly understand status throughout the entire project.
CAPTIS Modules
The 4 modules of CAPTIS include workflows to ensure your medical writing processes become more efficient:
See how CAPTIS can help you as a…
CAPTIS™ Features
With thorough knowledge of all aspects of medical writing, we offer an unparalleled end-to-end solution to ensure that you stay on track with post-market surveillance documentation.
Here are the features CAPTIS has to offer:
Source Document Management
Source Document Management
Information consistently represented across your PMS related reports;
Simplified review process for Notified Bodies.
Literature Search Database Integration
Literature Search Database Integration
Highly efficient literature reviews and reduction of manual effort with Literature Search Database integration and simple article management.
Adverse Event Databases Integration
Adverse Event Databases Integration
Integrated with TPLC, Maude and other adverse event databases for higher efficiency in adverse event analysis, especially when volume of data is high.
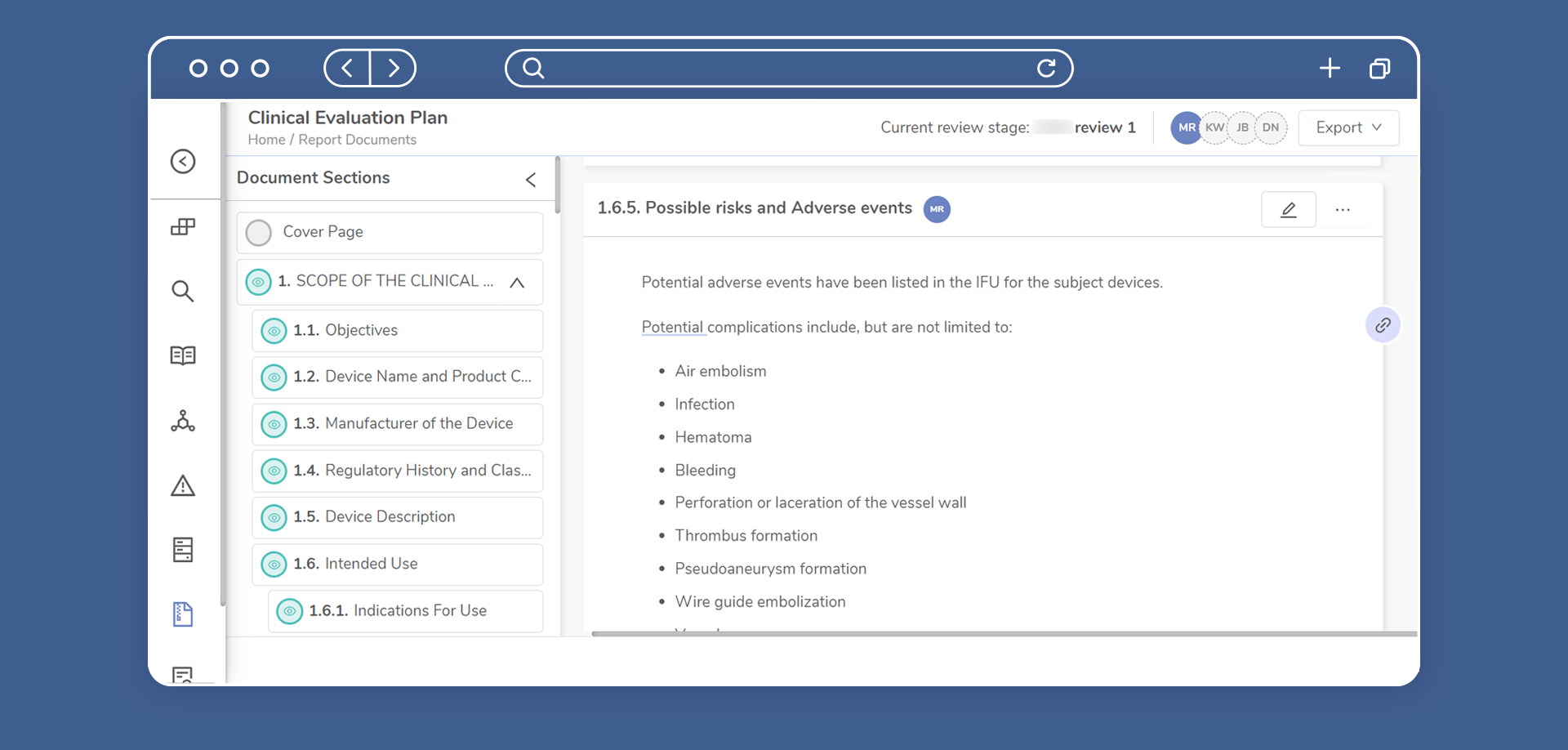
Article Audit Trails
Article Audit Trails
Full traceability of reviews and analysis conducted to support NB audits.
Predefined Templates
Predefined Templates
Maintain consistency across documentation by using predefined templates with relevant data required for your final reports.
Document Lifecycle Management
Document Lifecycle Management
Reduced rework and resource burden for ongoing maintenance activities of CERs, PMCF reports, PMSRs, etc.
Data Dictionaries
Data Dictionaries
Verbiage tied to specific words or phrases can be added to a master dictionary for easy re-use and consistency increasing speed and consistency in report writing.
Collaboration Tools
Collaboration Tools
Keep projects on track and ensure that the right information is shared at the right time among project managers, writers, and subject matter experts.
Bulk Import of Search Data
Bulk Import of Search Data
Cut time spent on collating data with bulk imports of search data.
Customizable Data Collection Forms
Customizable Data Collection Forms
Automatically updated summary tables thereby reducing human error
Mirroring sections in Document Creation
Mirroring sections in Document Creation
Sections or text that should remain consistent across different documentation gets mirrored to give you consistency across documentation.
Automatic Full-Text Retrieval
Automatic Full-Text Retrieval
Automatic retrieval of full-text articles saves time for medical writers.
Why Choose CAPTIS™?
| FEATURE | CAPTIS™ | OTHER SOLUTIONS | BENEFITS |
|---|---|---|---|
| Improved data retrieval via PubMed and Google Scholar | ✓ | ✕ |
|
| Option for separate but intertwined SoTA (State-of-the-Art) & DuE (Device under Evaluation) workflows | ✓ | ✕ |
|
| Customizable tables for summary reports | ✓ | ✕ |
|
| Source document management | ✓ | ✕ |
|
| Audit trail & reproducibility | ✓ | ✕ |
|
| US FDA MAUDE/TPLC adverse event database integration | ✓ | ✕ |
|
| Writing Support & In-Built Regulatory Intelligence | ✓ | ✕ |
|
| Content Linking when drafting Reports | ✓ | ✕ |
|
| Plug Dynamic Literature Review Tables into Reports | ✓ | ✕ |
|
| In-built writing support | ✓ | ✕ |
|
Subscription & Service Delivery Models
How can your team benefit from a CAPTIS™ subscription?
CAPTIS™ News
Redefining Compliance for EU MDR & IVDR
Maximize the Efficiency of your Medical Writing Team with CAPTIS™
Fill in the form now to get a 1-on-1 demo of CAPTIS and see how you and your team can benefit from it.
"*" indicates required fields