
How AI Speeds Up Systematic Literature Reviews by 60%
Systematic Literature Reviews (SLR), also known as Literature Search Reviews (LSR), play a vital role in ensuring compliance with stringent regulations such as the EU MDR. However, the traditional process of conducting SLRs, often performed by Medical and Clinical Affairs teams manually, is the most time-consuming and labor-intensive component of CERs. Enter AI-powered solutions, which are revolutionizing the way we approach SLRs, speeding up the process by an impressive 60%.
The Challenge of Systematic Literature Reviews
Systematic Literature Reviews are essential for compiling Clinical Evaluation Reports (CER) and meeting regulatory requirements. These reviews involve meticulously searching, screening, and analyzing vast amounts of scientific literature to ensure that medical devices are safe and effective. The process is not only time-consuming but also requires a high level of accuracy and attention to detail.
How AI is Transforming SLRs
AI-powered tools are changing the game by automating many of the labor-intensive tasks involved in SLRs. For instance, AI can quickly search and screen literature, identify relevant studies, and even extract data, significantly reducing the time and effort required. With a direct connection to databases, integration into the Literature Module, automations, simultaneous review by multiple reviewers, and other features, AI makes this tedious process immensely easier, taking the burden off SMEs.
Benefits of AI in SLRs
AI tools can process large volumes of data much faster than humans, reducing the time required for literature searches and reviews. AI algorithms can identify relevant studies with high precision, minimizing the risk of missing important information. AI ensures that the review process is consistent and reproducible, which is crucial for regulatory compliance. By automating repetitive tasks, AI reduces the need for manual labor, leading to cost savings.
Challenges Faced Before Using CAPTIS®
Before the introduction of CAPTIS®, our medical writers and regulatory professionals faced several challenges in conducting SLRs. The traditional process was highly manual, involving extensive literature searches, data extraction, and documentation. This often led to:
- Time Constraints: The manual nature of the process meant that literature reviews could take weeks or even months to complete, putting pressure on teams to meet tight deadlines.
- Data Management: Handling vast amounts of data from multiple sources was cumbersome and prone to errors. Ensuring the accuracy and consistency of the data was a significant challenge.
- Collaboration Issues: Coordinating efforts among team members was difficult, with feedback and updates often getting lost in lengthy email chains or disparate documents.
- Regulatory Compliance: Meeting the stringent documentation requirements of regulatory bodies required meticulous attention to detail, which was time-consuming and labor-intensive.
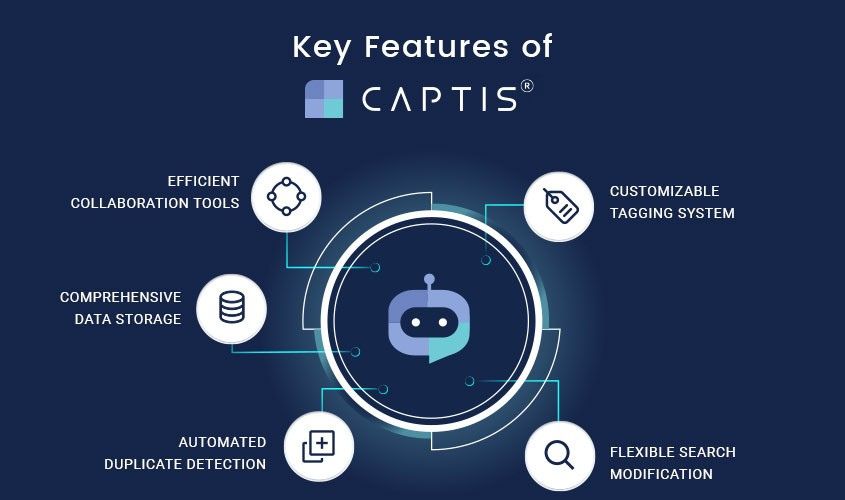
Celegence’s Approach to AI-Powered SLRs
At Celegence, we leverage our in-house AI medical writing platform, CAPTIS®, to streamline the SLR process. CAPTIS Copilot, an AI integration within the Literature module, automatically extracts relevant information from research articles such as study type, study subjects, demographics, interventions used, and adverse events. This information is summarized within seconds, allowing medical writers to be more efficient and consistently meet deadlines.
From literature search to screening and appraisal, CAPTIS® enables seamless collaboration and communication among team members. “From the literature search to the screening and appraisal performed on the platform and the documents that have been authored on CAPTIS, I can collaborate and communicate with the author and leave my feedback on the platform itself. The author can request for verification of actions performed and I can provide my updates after my review,” Celegence medical writer explained.
The data dictionary and automated reports generated by CAPTIS® make the review process more effective and the content more accurate. The content linking feature allows users to cross-verify data from source documents with just one click. Additionally, multiple reviewers can review a single document simultaneously, bringing efficiency to the process. “The features of data dictionary and automated reports that have been generated make the review process more effective and the content added more accurate. The content linking feature allows me to cross-verify the data that’s been added from the source documents in just one click. Multiple reviewers can review a single document simultaneously which brings efficiency to the process,” the medical writer added.
Conclusion
AI is transforming the way we conduct Systematic Literature Reviews, making the process faster, more accurate, and more efficient. By leveraging AI-powered tools like CAPTIS®, medical device professionals can ensure compliance with regulatory requirements while saving time and resources. Stay ahead of the curve by embracing AI in your SLR process. See how our AI-powered solutions can transform your regulatory processes. Contact us at info@celegence.com to learn more.


