
Regulatory Challenges in Writing EU MDR Compliant Clinical Evaluation Reports (CERs)
Welcome to the seventh post in our series of blogs related to some of the key challenges encountered by medical device manufacturers due to the recent updates to EU MDR. In this post we will focus on the Clinical Evaluation Reports (CER) and Plans (CEP). As of the latest regulatory changes, there is an increased emphasis on the detailed and transparent documentation of clinical data to ensure a thorough evaluation process. This includes stricter scrutiny on the sources and relevance of all kinds of clinical evidence as well as non-clinical or preclinical evidence used to support clinical documentation specially CEP and CER.
This post will focus on the EU MDR and Clinical Evaluation Reports (CER). Read our other posts about understanding:

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
Overview of EU MDR and Clinical Evaluation Reports (CER)
Production and distribution of medical devices in Europe is strictly governed by EU MDR regulations. It aims to ensure high standards of quality and safety for medical devices, replacing the previous Medical Device Directive (MDD) and the Directive on Active Implantable Medical Devices.
Key aspects of the EU MDR include:
- Stricter Clinical Evaluation Requirements: Devices must undergo rigorous clinical evaluations to ensure safety and performance.
- Enhanced Traceability: The regulation mandates a unique device identification (UDI) system to improve traceability.
- Post-Market Surveillance: Manufacturers are required to continuously monitor the performance and safety of their devices once they are on the market.
- Transparency and Documentation: Increased transparency through the European database on medical devices (Eudamed) and detailed technical documentation requirements.
These regulations are crucial for maintaining patient safety and fostering innovation in the medical device industry. A Clinical Evaluation Report (CER) is one of the crucial documents required under the EU MDR. It involves the assessment and analysis of clinical data related to a medical device to verify its clinical safety and performance. The CER must be continuously updated with new clinical data as it becomes available, ensuring ongoing compliance with the EU MDR.
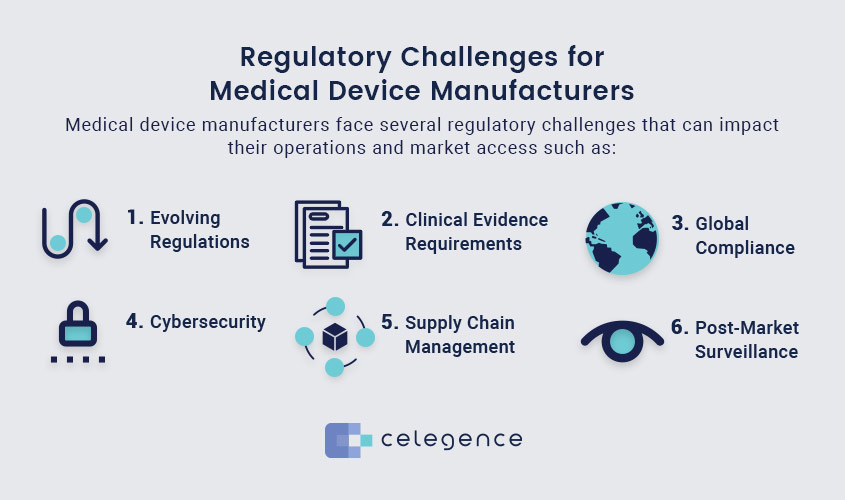
Regulatory Challenges for Medical Device Manufacturers
Medical device manufacturers face several regulatory challenges that can impact their operations and market access. They, like all businesses, expend their limited resources very carefully, making it difficult to invest equally in all products.
- Evolving Regulations: Regulatory frameworks, such as the EU Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR), are continuously evolving. Keeping up with these changes requires significant resources and constant monitoring.
- Clinical Evidence Requirements: Manufacturers must provide robust clinical evidence to demonstrate the medical device safety and efficacy. This often involves extensive clinical trials and data collection, which can be time-consuming and costly.
- Global Compliance: Different countries have varying regulatory requirements. Navigating these diverse regulations can be complex, especially for companies operating in multiple markets. For instance, the Medical Device Single Audit Program (MDSAP) aims to streamline audits across several countries, but it still requires thorough preparation.
- Cybersecurity: With the increasing integration of digital technologies in medical devices, ensuring cybersecurity has become a critical regulatory requirement. Manufacturers must protect patient data and device functionality from cyber threats.
- Supply Chain Management: Ensuring that all components and materials used in medical devices comply with regulatory standards is a significant challenge. This involves rigorous supplier audits and quality control measures.
- Post-Market Surveillance: Manufacturers are required to continuously monitor the performance of their devices once they are on the market as part of post-market surveillance requirements. Reporting of adverse events and implementation of corrective actions is also required under new provisions.
Addressing these challenges requires a proactive approach, including investing in regulatory expertise, adopting advanced technologies for data management, and maintaining robust quality management systems (QMS). Streamlining these processes is essential for medical device manufacturers to ensure efficiency and compliance.
New EU MDR Guidelines and Checklist
The new EU MDR guidelines are comprehensive and aim to ensure the safety and effectiveness of medical devices. The benchmark standard required to demonstrate continued safety and performance of subject device(s) has become more stringent when compared to the Medical Device Directive (MDD). Clinical Evaluation (MEDDEV 2.7.1) has been one of the key challenges under new MDR regulations. Under existing regulations, manufacturers have multiple options to demonstrate the safety and performance of their products. Clinical data held by manufacturer or clinical studies sponsored by manufacturer or literature are the primary sources that provide most of the required evidence for the device.
EU MDR Checklist mostly includes
- General Administrative Information
- Applicable Legislation
- General Safety and Performance Requirements (GSPRs)
- Clinical Evaluation
- Post-Market Surveillance (PMS) and Post-Market Clinical Follow-up (PMCF)
- Summary of Safety and Clinical Performance (SSCP)
The EU Commission has also introduced additional guidance documents to help manufacturers navigate the new requirements, including templates for clinical evaluation plans and reports that align with MEDDEV 2.7.1 Rev 4. These guidelines and checklist items are essential for ensuring compliance with the EU MDR and maintaining market access in the European Union.

Notified Body Requirements for Medical Devices
Notified bodies play a crucial role in the conformity assessment of medical devices before they can be placed on the market in the EU. They are designated by an EU Member State or other countries under specific agreements. They must meet stringent requirements related to organizational aspects, quality management, resources, and processes.
They assess whether medical devices comply with the MDR or IVDR which includes examining the technical design, testing the products, reviewing compliance documentation. Auditing manufacturer quality management system and performing products checks. Due to the difference in the type and risk of devices the process of audit becomes very complex. However irrespective of differences in price sales complexity of the products they still have to go through similar stringent rules for certification. The involvement of a notified body depends on the classification of the medical device. For example, Class I devices that are sterile, have a measuring function, or are reusable surgical instruments, as well as Class IIa, IIb, and III devices, require a notified body
Clinical Evaluation Report
A Clinical Evaluation Report (CER) is a comprehensive document that summarizes the clinical evaluation process for a medical device. It is essential for demonstrating the safety and performance of the device, particularly for regulatory approval in the EU under the Medical Devices Regulation EU 2017/745 (MDR).
These documents are mandatory mostly and are essential for manufacturers to obtain the CE certification. A Notified body shall audit all the CER except the class I products which are self-certified. The first important part of creating a CER is to prepare a CEP, which details the plan on how the evidence will be presented from which sources will they be obtained for the subject devices. Next step is to identify any clinical gaps in the clinical evidence for the subject device. Once done the quality appraisal and scientific validity of the clinical data is evaluated which is the third step. And the last but not least important part of CER is the evaluation of evidence that the device complies with GSPR.
Major important sections of CER are:
- Scope and Plan: Define the scope of the clinical evaluation and create a detailed plan outlining the objectives, methodology, and timelines
- Identification of Data: Gather relevant clinical data from various sources, including clinical investigations, scientific literature, and post-market surveillance (PMS) data.
- Appraisal of Data: Assess the quality and relevance of the collected data. This involves evaluating the scientific validity and applicability of the data to the device in question.
- Analysis of Data: Analyze the clinical data to determine the device’s safety and performance. This includes assessing the benefit-risk profile, identifying residual risks, and addressing any uncertainties or unanswered questions.
- Compilation of the Report: Summarize the findings in a structured report. The CER should include:
- General information about the device and manufacturer
- Description of the device’s intended purpose and technical characteristics
- Summary of clinical data and its appraisal
- Analysis demonstrating conformity with relevant safety and performance requirements
- Continuous Updates: The CER is a living document that should be regularly updated with new clinical data especially from post market surveillance activities.
Navigating Regulatory Challenges with Expert Assistance
Navigating regulatory challenges can be complex, but expert assistance can significantly streamline the process. Celegence offers tailored consulting services to help manufacturers navigate the complex regulatory landscape. Our experts provide strategic advice and hands-on support to optimize the clinical evaluation process. With the help of our experts, you can easily navigate through complex regulations, ensure global compliance and make sure your product meets all necessary standards for international markets.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.

Clinical Evaluation Reports (CER) Specialists
Our medical writing specialists in Clinical Evaluation process can provide invaluable support to medical by ensuring compliance with regulatory requirements and streamlining the CER preparation process. Our regulatory experts have in-depth knowledge of regulatory guidelines, such as the EU Medical Device Regulation (MDR) and FDA requirements and we ensure that the CER meets all necessary standards as per market standards. By leveraging the expertise of our CER specialists, manufacturers can ensure their clinical evaluation reports are thorough, compliant, and ready for regulatory submission.


