
ATMP Regulation in the EU and US: Global Convergence?
Advanced Therapy Medicinal Products (ATMPs) represent a breakthrough in modern medicine, offering new treatment potential for genetic disorders, cancer, and rare diseases. However, ensuring their safety and efficacy presents significant regulatory challenges.
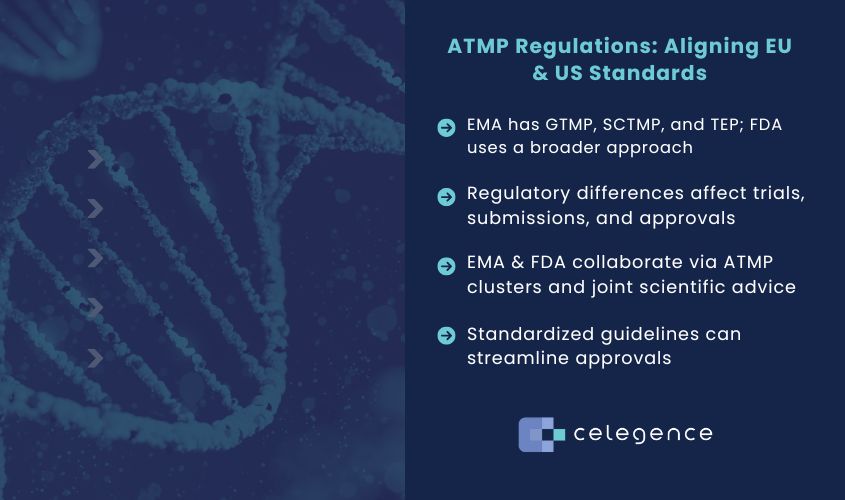
While the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) both oversee ATMPs, differences in regulatory approaches and classification frameworks can raise the bar for ATMP developers and slow down global market access. Efforts are underway to harmonize these regulations, aiming to streamline the development and approval of ATMPs across both regions.
Differences in Regulatory Approaches
The EU and US have distinct ways of categorizing ATMPs. The EMA employs a detailed sub-classification system, distinguishing between Gene Therapy Medicinal Products (GTMP), Somatic Cell Therapy Medicinal Products (SCTMP), and Tissue Engineered Products (TEP). This nuanced approach helps address the specific characteristics and mechanisms of action for each therapy type, even though all subtypes are governed by the same overarching directive as other classes of medicinal products. In contrast, the FDA follows a broader framework, primarily focusing on gene and cell therapies without the same level of granularity. While both agencies require ATMPs to involve genetic modifications, engineered cells, or tissue manipulation, the different classification systems can create challenges for companies seeking approval in both regions.
Another key challenge arises in the evaluation process for somatic cell therapies, which have a wide range of therapeutic applications. The FDA and EMA sometimes differ in how they classify and assess these products, which can influence how companies design clinical trials and plan regulatory submissions. Nevertheless, regardless of the classification, a full regulatory dossier is required to support an EU or US application.
Collaborative Efforts Toward Harmonization
Recognizing these challenges, the EMA and FDA have been working together for over a decade to enhance regulatory consistency. One significant initiative is the formation of an ATMP cluster under the EU/US collaboration on medicines. This cluster facilitates knowledge exchange between regulatory bodies, helping to address scientific and procedural challenges in ATMP regulation.
Despite this progress, notable differences remain. The FDA has issued disease-specific guidance for certain ATMPs, such as those targeting haemophilia and retinal disorders, while the EMA takes a more general approach, covering ATMPs as broad categories. This discrepancy can lead to different regulatory expectations for manufacturers, making it difficult to navigate approvals in both regions.
Steps Toward Better Convergence
To reduce these regulatory barriers and promote alignment, several steps can be taken:
- Developing Common Guidelines: Establishing shared regulatory guidelines that encompass the full range of ATMPs would provide greater clarity for ATMP developers and streamline approval pathways across the EU and US. This would also help ensure that therapies developed in one region meet the standards of the other, facilitating global patient access.
- Improving Parallel Scientific Advice: Increasing the number of joint scientific advice sessions between the EMA and FDA would allow developers to receive early-stage guidance from both agencies. This approach helps clarify regulatory expectations, reducing uncertainties and potential delays in the approval process.
- Aligning Post-Market Surveillance Practices: Both agencies emphasize the importance of real-world evidence and post-market monitoring for ATMPs. By harmonizing data collection and analysis frameworks, the EMA and FDA can ensure consistent safety and efficacy assessments across regions.
Future Outlook
While regulatory differences persist, ongoing collaboration between the EMA and FDA signals a promising shift toward global harmonization of ATMP regulations. The creation of joint initiatives, shared scientific frameworks, and improved regulatory dialogue will help reduce inefficiencies and foster a more predictable approval landscape for ATMPs.
By aligning standards, increasing cooperation, and optimizing regulatory processes, the EMA and FDA can facilitate speedier development and commercialization of ATMPs. These efforts will ultimately benefit patients worldwide, ensuring timely access to innovative therapies while maintaining the highest quality standards and positive benefit-risk balance.
Interested in the CMC challenges ATMP developers face and how to address them? Read Part 2: CMC Challenges & Regulatory Strategy for ATMPs & CGTs.
Want practical tips on reducing CMC timelines and mitigating regulatory risks? Explore Part 3: Reducing CMC Timelines & Risk in ATMPs & CGTs.
For more information on how Celegence can help improve your regulatory compliance, reach out to us at info@celegence.com, contact us online or read more about Celegence’s services.


