Case Study: Medical Device Registration Project in India, Bangladesh, Pakistan and Sri Lanka for a Global Medical Device Company
Background – Client Needs:
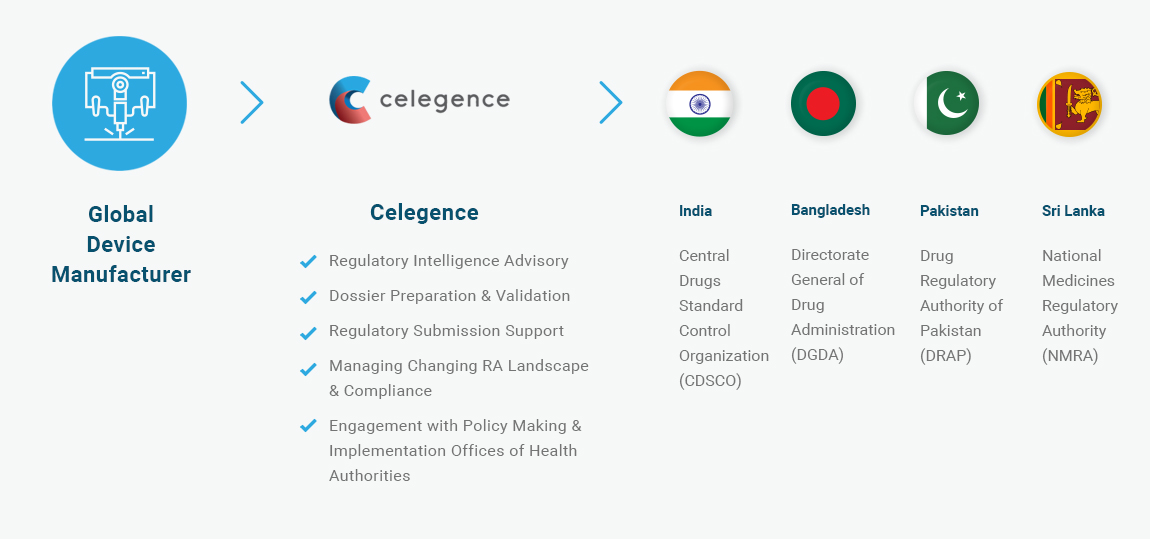
A US medical device manufacturer was looking to introduce a new medical device and expand its market in India and neighboring countries (Class IIa and IIb). The manufacturer sought regulatory support in submission and obtaining approval to market these new products for business expansion.
The client faced the following challenges:
The scope of Celegence activities was to manage all regulatory activities and ensure full regulatory compliance for medical device registration in India, Sri Lanka, Pakistan, and Bangladesh. The team was expected to work closely with local regulatory authorities to ensure the latest regulation requirements regarding product registration and maintenance were addressed. The team was also expected to engage in regular project monitoring, documentation filing, and timely status reporting.
Project Initiation & Key Objectives:
The client chose to leverage Celegence’s team of medical device regulatory experts to support this Medical device registration project based on our extensive expert network and ability to complete the project in a cost-efficient and timely manner.
The client’s objectives involved realising the following business benefits via their outsourcing strategy:
Celegence Solution & Approach:
Celegence was awarded a contract to provide support in Medical Device Registration services and performing submission and obtaining approvals for new medical devices as per countries guidelines, including:
The initial information was provided by the Celegence delivery team with prior experience of working closely with Regulatory authorities. The checklist and other information were gathered as per the regulatory guidelines including the following:
The output of the task was on-time preparation of the submission dossier for the assigned medical devices including the strategy. Key to the success of the project was our team’s integration, in house medical device experts, and direct contact with health authority representatives.