
Ensuring Compliance for your IVD’s Performance Evaluation – Webinar Transcript – Part 1
Joseph has 20 years of experience in medical device life cycle management and quality management systems (certified to ISO 13485, ISO 9001 & ISO/IEC17025). His key expertise includes the preparation and maintenance of technical files, product safety and vigilance reporting, clinical evaluations, risk assessment, regulatory audits, CE and notified body opinion submissions. Medical devices industry – Joseph has experience in diabetes management devices, nicotine replacement therapy (NRT) devices and pressurized metered dose inhalers (pMDI). Joseph has worked for small, medium, and large Biotech companies such as Roche, OBG Pharmaceuticals, and Kind consumer Ltd.
Webinar Background:
The new in vitro diagnostics regulation of the European Union, the IVDR 746/2017, will soon be replacing the existing IVD Directive on May 26th, 2022. The regulation is set to bring in many drastic changes to the way the IVD medical devices are regulated by strengthening the old requirements and adding many new ones.
The IVDR changes have strengthened the requirements for Performance Evaluations, which are conducted in the premarket phase. Further, it mandates that the performance of devices be continuously monitored throughout the life cycle of the device. The IVDR requires the continuous data collection, analysis and drafting of a Performance Evaluation Report (PER).
During our webinar titled “Ensuring Compliance for your IVD’s Performance Evaluation” Joseph Richardson Larbi, spoke specifically about his perspective on the essential components of a PER and how to prepare key technical documentation under the EU IVDR and what manufacturers must do to stay compliant.
Why should you read the transcript/watch this video?
- To best understand the performance data requirements under the EU IVDR for IVDs
- To Learn how the requirements could affect your current product lifecycle approach/QMS
- To examine how to apply the industry best practices for systematic literature reviews
- To find out the common pitfalls to avoid when drafting a PER for a smooth notified body or competent authority submission
A full transcript of Joseph’s presentation is available to download (and to read below) and just press play to watch the clip now.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
IVDs – History and Regulation Timeline
What are IVDs? – Article 2 Section 2 has got the full definition of an IVD medical device. IVDs are devices that analyze samples away from the body or out of the body. If you draw a blood sample and you are going to test in a laboratory, these devices which are software control solutions, calibrators are all classified as IVDs.
In terms of the history of how medical devices have been regulated to date. There was the old approach, especially in Europe, this was pre 1990s and then the European Commission or Council decided to come up with a new approach of directives. This is where we saw the medical device directive coming up. The active implantable medical device directive was coming out and then the In Vitro Diagnostic directive was coming out.
The active implantable directive (AIMDD 90/385/EC) was the first that was released under the new approach of regulating medical devices as early as 1990. These directives have been around for some time.
The medical device directive (MDD 92/42/EC) came about in June 1993 and then the IVDs (IVDD 98/79/EC) was released in 1998. These three devices formed the new approach of regulating medical devices within the European region.
Since 1998, the IVDs have been in place, and we all know what has happened within the medical devices space in terms of metal or metal hip implant using industrial grade Silicone for breasting plants and the issues that it’s caused to some patients and dodgy notified bodies that were out there not really doing things as they should. Manufacturers also not keeping good records and so on and so forth which all went into sort of like a spiral effect and caused the European Commission to really look into how medical devices are being regulated and decide to publish a new set of regulations.
So the difference is directives, which were you could pick and choose certain elements and then make it into a national law or a statutory instrument for the UK. The difference is for a regulation you have to straight away it’s the law. You have to adapt and implement straight away. So that’s where we find ourselves at the moment – the medical devices have actually applied their regulation. So that was on the 22nd of May 2021. And for the IVDs, we are looking at the 22nd of May 2022.
IVD Timeline and Transition to the New Regulations
The directive of the IVD was used from 1998, all the way to 2017 or will be used all the way to 2022. It’s about time that we revamp these directives and go to something else that will match the current technology and what’s happening in the medical device industry.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
Key Changes to IVDR from IVDD
The date of application for the IVDR is the 26th of May 2022. However, about a month ago, the European Commission submitted to the parliament and the council a proposal to postpone the date of application for the IVDR. It is planned that if this is accepted, the transitional period for certain devices is going to change.
Looking at key changes that are changing between the IVD directive going into a regulation, you have a shift in regulatory scrutiny. The notified bodies are all being reassessed. So under the directive you had 19 or 20 notified bodies at the moment we have only got 6 notified bodies designated according to the regulation, which is part of the reason why the European Commission is seeking an extension to the date of application. Looking at what’s happening with COVID and in the middle of this pandemic and what you need is a groundbreaking technology.
The IVD directive was basically list based when it comes to classification, which is one big thing that’s changing under the regulations where the directive was list A/B. Now the regulation is going into a rule-based classification. So class A – D, from lowest to highest in that order A to D and when it comes to devices, that will come under the oversight of a notified body. So what you can set self-certify on what you cannot self-certify. There’s a big shift. Under the directive about 10 – 15% were under a notified body oversight that needed certification. The majority of IVD’s under the directives were self-certified. So you had a good 85% of all IVDs being self-certified. Whereas now it’s flipped the other way around, a good 85 – 90% will fall under the responsibility of the notified body. It’s not that we have only got fewer notified bodies designated to the new regulation, but now you have got an increase in products that are actually going to the notified bodies as well.

Key Changes – Possible Postponement
Class D devices will be extended until 2025. So that’s an additional one year for a high-risk class, because currently that’s May 2024. For a class C that will be in May 2026. So that class C will have a two-year extended period. Class B and A will be extended to 2027. So they will then have a three-year gap.
Key Compliance Elements of the IVDR
Let’s quickly go into key compliance elements of the IVDR, which also entails the performance evaluation reports and then we’re going to performance evaluation reports. Have a look at some best practices, the best way to draft your performance evaluation reports.
The classification is key to the IVDR. So the first thing is to reassess, where your devices fall from itself certified IVD under the directive and it might fall into a class A or B, or some of them could jump all the way from self-certified to a class D so nothing is impossible here. So just go through the classification rules, article 47 or Annex VIII of the IVDR and make sure that your class is right.
Have a look at the obligations for manufacturers, that’s in article 10 of the IVDR and bear in mind that the IVDR is a lifecycle approach. So continuous compliance. It doesn’t stop after you have submitted your technical file and got your CE mark approval for your IVD’s. You have to continue to monitor its performance, submit reports to the competent authorities or to the Notified bodies depending on your classification and the reports that you need to submit whether it is PSUR or PMS report or a Performance Evaluation report or post market performance follow up report. So all of these things. So just bear in mind that it is a continuous compliance journey that we are on, not just a one time thing.
For your device identification and registration. So now you’ve got UDI’s and EUDAMED, which is now active. So now actors, manufacturers or distributors can register themselves in EUDAMED and you can actually start registering devices as well.
So the second and third module of EUDAMED is now open. So if you’re not familiar with that, have a look at the European Commission’s website and familiarize yourself with it and start registering your devices accordingly.
Then we’ve got clinical evidence. So clinical evidence will all come about from your performance evaluations and your performance studies. Make sure you submit good clinical evidence for your IVDs.
We’ve only got 6 notified bodies at the moment. You can also find this list on the EC NANDO website.
Conformity Assessment Routes
For class A which is going to be self-certified, you have to prepare technical documentation according to Annex II and III of the IVDR, and then you cannot fix yourself a CE mark on the device or its packaging.
For certain classes that are sterile, class B, C and D; Then you’ve also got their roots for certification as well and you can see the difference in the CE marks there you do have a notified body identification number underneath the CE mark. So just better in mind, also for class Ds you will need an expert consultation as well it is stated in article 48 of the IVDR. So do have a look at those and make sure that you’re complying as well.
Performance Evaluation Report (PER) of an IVD
Standards/Guidance Documents Needed to draft a good PER:
You have got Annex 56 and Annex 13 of the IVDR. You have got Annex I of the IVDR. Basically Article 56 points you to certain elements of Annex I that is the GSPR of the IVDR. You have got guidance documents provided by the Global Harmonization Task Force (GHTF) which is now the International Medical Device Regulators Forum (IMDRF).
These particular documents for IVDs were not transposed into IMDRF. There is the N6, N7 and N8 of these particular documents. Have a look at those as well. N6 refers to definitions, N7 goes into depth, telling you the things that you need to consider for the elements of your performance evaluation report and N8 goes into clinical performance studies. So if you have to carry out a clinical performance study, then the standards and practices that you need to follow.
MEDDEV 2.7.1/R4, that’s solely for medical devices, but also applies to IVDs because an IVD is technically still a medical device. So there are things that we can draw parallels from.
We have got the MDCG 2020-1: PER for MDSW that’s for performance evaluation report for medical device software. When it comes specifically for the IVD’s, there isn’t much under the MDCG for IVDs, unless you’ve got something solely related to COVID 19. In that case, there are a lot of guidance documents under the MDCG for devices and performance evaluations for devices that are testing for COVID 19 related stuff.
What is a Performance Evaluation?
There are three elements of a performance evaluation, and that is the scientific validity of the analyte that you are analyzing, the analytical performance and your clinical performance. So these are the 3 main pillars of a performance evaluation.
So a performance evaluation is an assessment and analysis of data to establish or verify the scientific validity, the analytical and way applicable the clinical performance of a device.
Scientific validity of an analyte, this is basically the association of analyte with a clinical condition. So how can you prove that testing a blood sample can give results for a particular clinical condition? For example, if we take people with diabetes, then they could test blood samples to test blood glucose, but what is to show that testing blood glucose will give you the analysis of how high or low they are in blood sugar.
Analytical performance is the performance of the device itself to actually detect the analyte that you are intended to measure. And once you’ve got that, then you’ve got your clinical performance. How do these test results relate or correlate with a particular clinical condition? so these are the three main umbrellas of a performance evaluation.
Now these three pillars are then wrapped into a few other things. So you can wrap these three as one bundle and you can wrap them up with state of the art. So all these things that you are doing, you are supposed to compare with the state of the art in medicine of that particular field that you are in. You analyze your risk elements, what’s your benefit risk ratio of your device when used as intended by the manufacturer? So that’s one thing and how does it demonstrate compliance to the GSPRs? So that’s another thing. So these things, when you wrap them all one, this is your performance evaluation report.
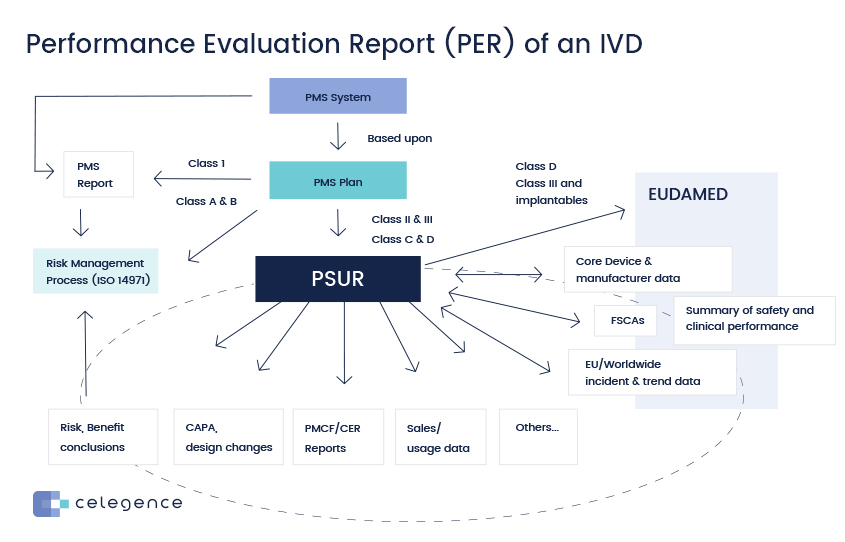
Performance Evaluation Report – Concept/Process
You have got your clinical performance, scientific validity, and analytical performance as your big circle. You have to work with the state of the art. When you draw in data, you must draw data from your post market surveillance system. You must also draw data from your post market performance follow up that feeds into your PER. You must consider your risk management approach as well. You should work with the recent ISO standards in risk management ISO 14971, specifically 2019. That’s the latest standard that’s out there at the moment.
Performance Evaluation Report for IVDs
When I am drafting the PER, I’ll start with a device description and it’s very important to lay out what the device is about. What is it doing, what are the manufacturer’s intentions for the device? So the reader can understand the device before it goes into the scientific validity, the analytical performance, and the clinical performance. It makes it easier for the reader or the notified body to understand your report, if you report it this way.
So start your report in a way that’s easily understood by the reader. I will go into the intended use and the purpose of the device. You’ll be surprised if the number of reports that I review, and it’s not clear, or it hasn’t been stated clearly what the intended use is, and this is under the IVDD. Note that the IVDD didn’t specify for intended use, but there are certain practices that certain manufacturers have adopted, and it has gone unnoticed, and they don’t clearly state what the intended use or the intended purpose is from certain literature that I review which is not right.
So always make sure you state clearly what the intended use or the intended purpose is. The targeted population that the device is supposed to be using whether it’s pediatrics or adults. If you specify a particular age, make sure that you state those, if it’s for a particular stage in a clinical condition, then make sure that you do state the stage of that particular clinical condition as well.
If it’s in the early stages or the advanced stages, make sure you do state all of this – who the intended user is? is it for the actual patient? is it for the healthcare professional? Make sure all of these things are stated. The use environment or the location for testing – is it in the laboratory, is it at home, in the hospital by the bedside? Make sure all of these things are stated.
Your risk classification – the new classes under the performance evaluation – class A, B, C & D. Make sure you state clearly what the classification of the device is and the rationale behind the classification. A key thing that the notified bodies under the new regulations want to see, especially when it comes to the classification, is not just saying it’s rule number 4 under the IVDR. They want to see you demonstrating how you came to that risk classification. So they want to see you are analyzing all applicable rules of the IVDR, and why you decided to select the particular one that you’ve ended up with.
Device characteristics – what performance are you claiming for your device? Make sure that is clearly stated in the performance evaluation report. Your performance parameters or specification, whether its diagnostic sensitivity or specificity, make sure you state these things clearly like diagnostic sensitivity is to detect that an analyte is testing a particular condition and specificity is opposite of that. So make sure that all of these things are stated clearly within your performance evaluation report.
Make sure you describe the analyte or the marker that you are analyzing. The technology involved in the device, make sure you elaborate on that. Any limitations of your device and contraindications, things that the device should not be used for. All of these things fall under the device description.
Stability parameters – the claimed shelf life, In-use stability, and shipping stability. Temperature controls and storage, all of these things make sure that you state these clearly. By the time you’ve listed all of these things in the report to a very robust level, it makes it easier for the notified body by the time they get into the actual clinical evidence or the clinical data that they’re about to review. It also gives them a good impression of the manufacturer and they know that they are off to a good start and they’ll be in a good mood, and they’ll be able to review and provide some good feedback.
Performance Evaluation Methods
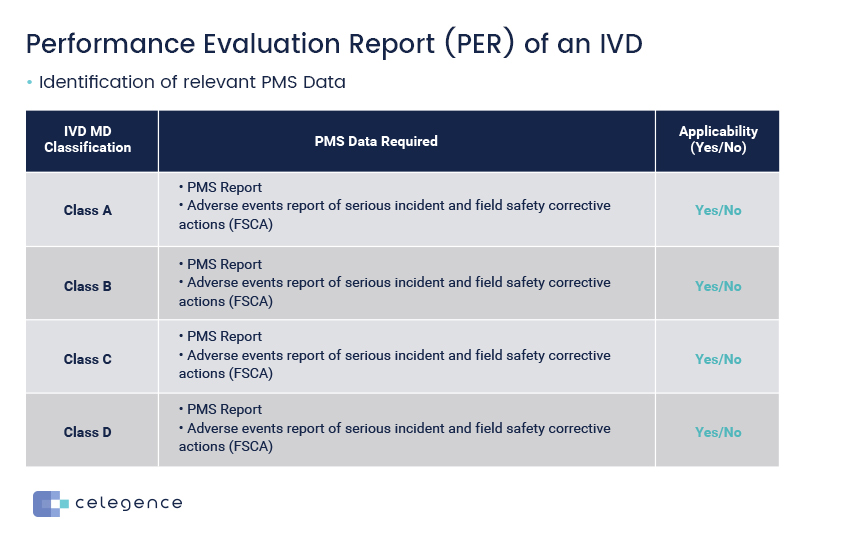
PER is to analyze your clinical evidence and the clinical evidence is really the three things scientific validity, analytical performance, and clinical performance. You want to demonstrate that the clinical evidence that you are gathering, the data that you are gathering from these three parameters has clinical benefits. So your device is going to improve a patient’s life or to make the work of a healthcare professional easier. To demonstrate that the clinical evidence has clinical benefits, demonstrates compliance to the relevant GSPRs. In addition to this, the device must have an acceptable benefit-risk ratio. So you must also demonstrate that your device when used in the environment as intended by the manufacturer, and if you follow the instructions as per the manufacturer will have an acceptable benefit risk ratio. So the benefits of using the device must outweigh any risks, any harm to the patient and you must be able to demonstrate this in your PER. So when you are considering all of this data, you must consider data from your post market surveillance and post market performance follow up as your PMS plan.
Identification of relevant PMS Data
Celegence’s comprehensive support for creating and maintaining required PMPF documentation can help ensure your organization’s IVDR compliance. Reach out to us at info@celegence.com to learn more, contact us online or read more about Celegence’s medical device services.


