
Data Dictionary – CAPTIS™ Features
“…let me just transfer all this device information from the Clinical Evaluation Plan to the Clinical Evaluation Report template. Quite a few sections are the same.” Sounds familiar?
The arrival of the EU MDR and IVDR introduced new reporting requirements for medical device manufacturers. If you’re a part of a team authoring regulatory documents for the EU MDR/IVDR, terms like the CEP, CER, CPR, APR, SVR, PEP, PER, PMSR, PSUR, SSCP and PMCF must be familiar to you.
And once you’ve authored multiple reports (for MDR or IVDR), you will realize that quite a few of the sections between these reports are just about the same. Did you ever wonder, “Why can I not leverage the same section for another report?” Well, we did which gave rise to our Data Dictionary feature in CAPTIS™.
Repeating Information in Regulatory Documents
There are often multiple report documents that must be created for the same medical device/device family per the EU MDR/IVDR Requirements. A few of these MDR and IVDR reports include:
- Clinical Evaluation Plans (CEP)
- Clinical Evaluation Report (CER)
- Clinical Performance Report (CPR)
- Analytical Performance Report (APR)
- Scientific Validity Report (SVR)
- Performance Evaluation Plan (PEP)
- Performance Evaluation Report (PER)
- Post-Market Surveillance (PMS) Plan
- Post-Market Surveillance Reports (PMSR)
- Periodic Safety Update Report (PSUR)
- Summary of Safety and Clinical Performance (SSCP)/Summary of Safety and Performance (SSP)
- Post-Market Clinical Follow-up (PMCF) Plan/Post-Market Performance Follow-up (PMPF) Plan
- Post-Market Clinical Follow-up (PMCF) Report/Post-Market Performance Follow-up (PMPF) Report
While the bulk of the report content remains unique across these reports, there are certain sections and blocks of information which remain the same and are repeated throughout. For example:
- Device Description
- Product Formulation/Material Composition
- Principle of Operation/Mode of Action
- Intended Use of the Medical Device
- Indication(s)
- List of part numbers/SKUs in scope of the Report
- Manufacturer Details
- Regulatory Information on the Device
- Market History
- Safety and Performance Claims
- Year of Launch
- Year of CE mark approval
- Technical File Number
Additionally, these reports may have information which is presented at multiple instances within the same report – the date range, overall complaints rate, sales numbers, number of hits from literature, the number of included articles, and the number of reported adverse events, to name a few.
The checklist highlights all of the documentation that you will need in place for certification of your IVD device and will serve as a guide to help you achieve ongoing compliance. In conjunction with this checklist, we are also able to provide you with bespoke strategies to bring your business up to speed. We are currently working with businesses from the United States, India, and throughout Europe to ensure that they are ready for the deadline in May of 2022.
The Challenge
Recurrent information which needs to stay consistent throughout, presents unique challenges in maintaining consistency across all occurrences where the information is presented. Take the date range in a near complete CER for instance; changes in the search strategy for device-specific literature are quite often seen in terms of expansion of the date range set initially. This translates into scanning and updating all occurrences of the date range in the CEP, CER, the corresponding Literature Search Report, PMSR/PSUR and any other documentation which leverages that literature search.
Now think bigger, consider a major change in the device description. Think of the different documents that will be impacted and will require an update to maintain consistency. The scope of work may seem simple, but the effort required to implement the change, i.e., identifying which documents use the information, making the said change, and verifying whether all documents have been appropriately updated, is significant.
The Solution – Data Dictionary in CAPTIS™
At Celegence, we understand operational challenges faced by device manufacturers and medical writers alike and use technology to overcome these challenges, like the PRISMA Flow Diagram feature in CAPTIS™. Hence, we developed the Data Dictionary feature to add into CAPTIS™ too.
The Data Dictionary acts like a master repository where segments of text can be saved and easily called upon. You can use the Data Dictionary to save information that will be repeated throughout your documents and populate this data into your reports by using simple commands from your keyboard or through the CAPTIS™ interface. And what’s more, any updates to the master information can be made via editing the saved entry, and all occurrences of the linked entry will be auto updated within the same report, and even across different reports.
The Data Dictionary feature can also be configured in a way to auto populate reports. Once you configure your report templates on CAPTIS™, you can use the Data Dictionary to pre-fill a linked report even before you draft the actual document, and thus, save time by only working on sections which are unique to the report.
How Data Dictionary Master Repository Works
Once you set up entries in the Data Dictionary within the CAPTIS™ platform, the dictionary can be accessed when editing report sections on CAPTIS™.
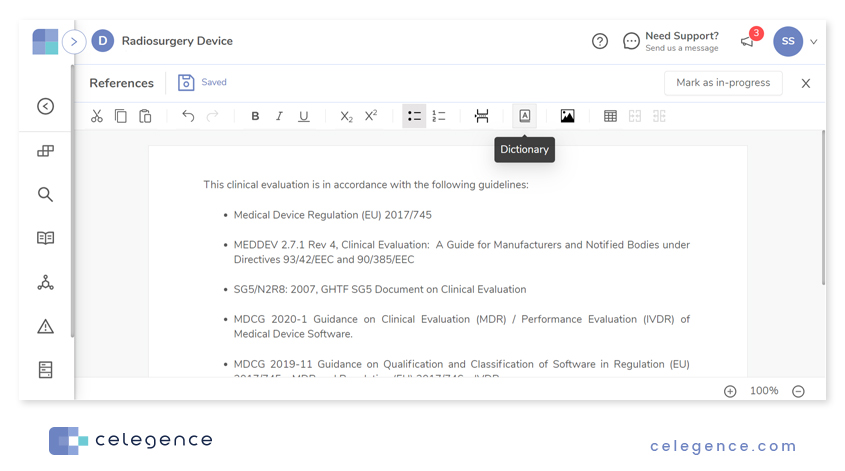
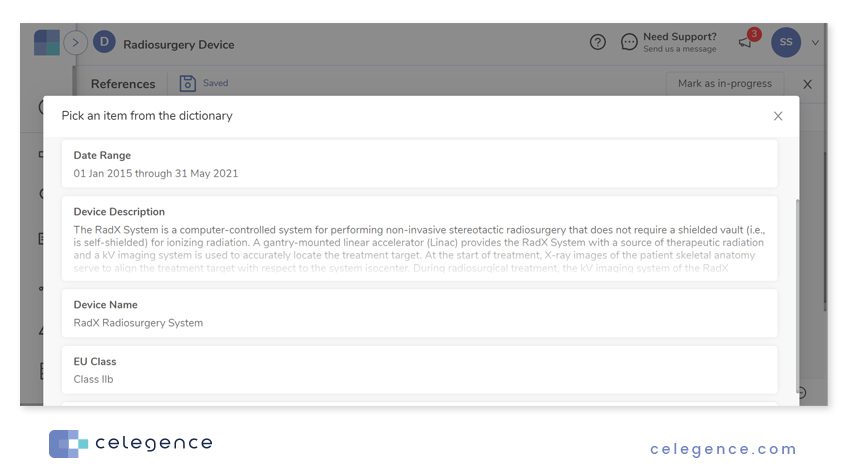
On clicking the Dictionary icon, CAPTIS™ presents a list of all saved dictionary items. Click on the entry of choice to use the entry in the current section.
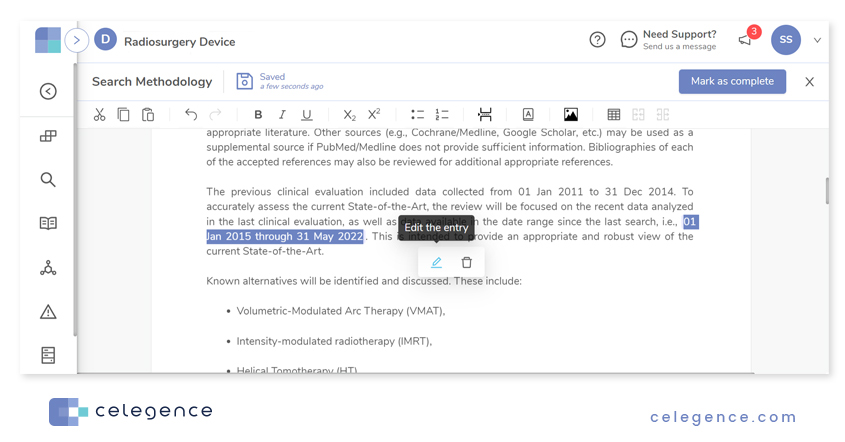
Adding repeating text via dictionary entries ensures that your report content stays consistent. Changes to the master entry can be done by simply editing the entry in place. Consequently, all occurrences of the entry will be updated.
Key Pain Points Without the Data Dictionary or Master Repository
| Pain Point | Description |
|---|---|
| Data Repetition | Repeating data across multiple reports. |
| Prone to Error | Change in the master data from the Technical File leads to sequential updates in all related reports. Manual tracking methods to identify, update and verify the change can often be error-prone. |
| Cumbersome | Updating repeating data within the same report involves manually searching for the initial data, followed by updating any verifying each occurrence. |
Advantages of using the Data Dictionary within CAPTIS™ for Report Authoring
Here are some of the benefits of using the Data Dictionary when creating reports on CAPTIS™:
- Keep your primary device data stored in a master repository and plug it into various reports in an instant
- Standardized sections across EU MDR/IVDR reports
- Time and effort savings by letting CAPTIS™ auto populate sections in linked reports
- Quick and easy updates to the master entry
- Improved consistency within reports by having CAPTIS™ automatically update all occurrences of a repeating data entry
Schedule Your CAPTIS™ Demo
Your medical writing team can benefit from CAPTIS™ with faster turnaround times for systematic literature reviews and more accurate end-to-end MDR/IVDR documentation support. To learn more and view a comprehensive demo of CAPTIS™, reach out to info@celegence.com today or contact us online to connect with a Celegence representative.
The checklist highlights all of the documentation that you will need in place for certification of your IVD device and will serve as a guide to help you achieve ongoing compliance. In conjunction with this checklist, we are also able to provide you with bespoke strategies to bring your business up to speed. We are currently working with businesses from the United States, India, and throughout Europe to ensure that they are ready for the deadline in May of 2022.



