Celegence enables companies to author Right-First-Time (RFT) documents using the full set of Dosscriber™ eCTD document templates (other formats also available, e.g., ASEAN). The document templates optimize lifecycle management, ensure consistency, and avoid duplication of work.
From our extensive experience with pharma clients, we have put great effort into avoiding document duplication. Therefore, we created eCTD templates to ensure accuracy, consistency, and reusability of documents across dossiers.
Easy-to-use eCTD Document Templates
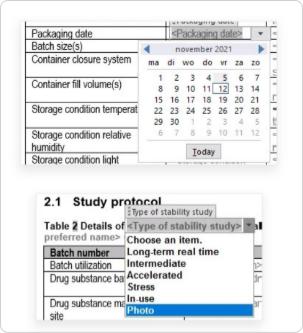
Dosscriber™ features content control boxes and pick lists to ensure your content complies with regulatory standards.
Reusability and Reduced Authoring Time
One of the foundational pillars of our eCTD templates is separating content from context. This concept is key to ensuring document reusability and saving time when creating global dossiers.
Tabular Formats for Lean and Structured Authoring
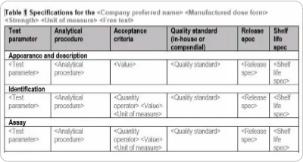
Our common technical document templates apply tabular formats or bullet lists for factual information, where possible. Additionally, placeholders are used for factual values, and content control boxes are aligned with IDMP implementation guidelines.
Embedded Font and Paragraph Styles
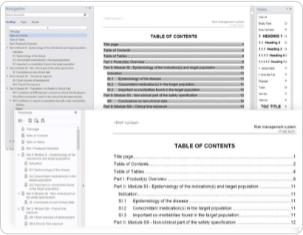
Dosscriber™ supports the automatic creation of TOCs, PDF bookmarks, and cross-references, which results in optimal navigation and significantly reduces the time required to publish to eCTD format.
Multiple Options for Granularity
For each eCTD subsection, our templates offer support for both coarse and fine granularity (single- or multiple documents). This provides flexibility when outlining your submissions.
Prefilled Document Headers and Footers for Global Submissions
eCTD templates include document header information and prefilled naming properties, ensuring consistent use of metadata. These are designed to optimize reuse without rework across products, countries, and strengths.
Preformatted Narrative
Standardized narratives are included for key eCTD sections, providing the right amount of detail across documents, sections, and dossiers.
eCTD Submission-Ready Documents Directly After PDF Conversion
Dosscriber™ allows you to create documents that are 99% submission-ready upon completion. Features like bookmarks, hyperlinks, and TOCs are automatically generated by following heading styles, reducing the time required for PDF publishing and speeding up dossier compilation.
eCTD Document Templates for All Regions
Considering that Module 1 of eCTD dossiers is country-specific, we have created Module 1 eCTD templates tailored to each country/region.
Compliance with Up-to-date Regulatory Guidelines
Hidden regulatory guidance is provided to help authors, sourced from ICH guidelines, and enriched with insights from recognized experts, including former MHRA assessor and ICH representative Anthony C. Cartwright. Our templates are regularly updated to remain compliant with the latest regulatory standards.
Celegence Best Practice Guidance for Document Authoring in eCTD Format
Practical experience is included as hidden text or in separate manuals for CTD modules and subsections (e.g., Module 2, 32P, and 32S). Hands-on training is provided to ensure users are familiar with the Dosscriber™ templates.