Overview of eCTD Submission Modules and Format
It would be a vast understatement to say that new drug and biologics applications are “complicated.” New submissions can include hundreds or thousands of documents. So, it’s not hard to figure out why regulatory reviewers wanted to standardize the submission format, cross link documents within submissions and make it fully digital.
Regulators from the US FDA Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) now shun binders and require that new drug and biologics applications be submitted in Electronic Common Technical Document (eCTD) format. eCTD is simply the electronic version of a CTD submission. Other markets that have implemented eCTD include include Australia, Brazil, Canada, China, Europe, GCC countries, Japan, Jordan, Singapore, South Africa, South Korea, Switzerland, Taiwan, and Turkey.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
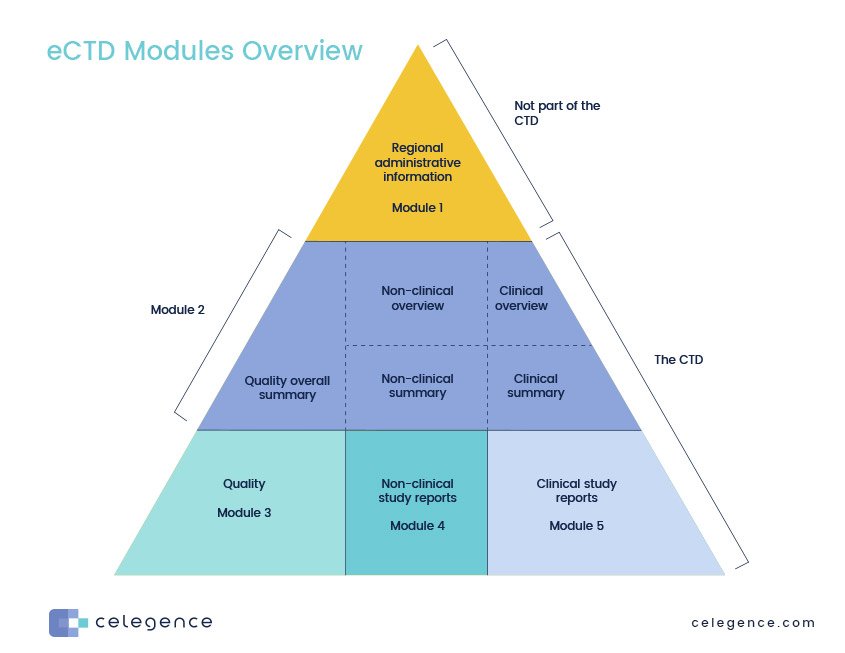
eCTD Modules Overview
Fortunately, all these market regulators have agreed on a common standard for exchanging regulatory information between industry and agency in eCTD format. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) coordinates eCTD standards for compliance.
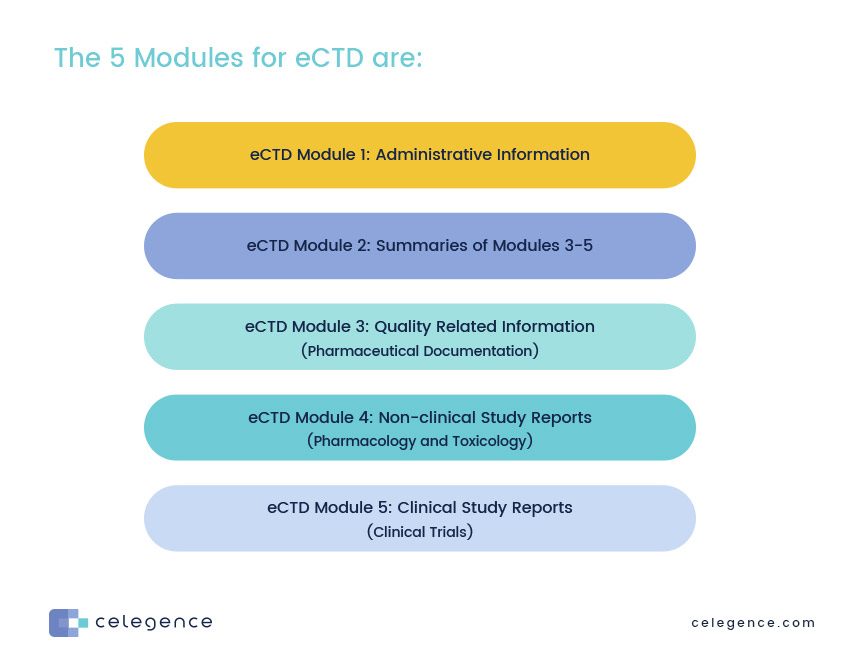
Let’s take a high-level look at the eCTD format. It has five basic modules as shown below.
eCTD Module 1: Administrative Information
This module is specific to each market regulator and is not part of the CTD. For example, not surprisingly the US FDA has created dozens of forms over the years, and they require specific information for each submission. The same can be said for regulators in Australia, Canada, Europe, and other markets that use eCTD. This module allows those regulators to collect and track information that may be extraneous to the submission but necessary for their specific systems.
eCTD Module 2: Summaries of Modules 3-5
This module is the true beginning of an eCTD submission which is one major component of a comprehensive regulatory dossier. The eCTD contains an introduction, overall quality summary, non-clinical and clinical overviews, and summaries. It is here that the applicant adds the Quality Overall Summary (QOS) – a series of overviews of key data contained in Module 3. The QOS will cover key issues and emphasize important parameters of the drug or biologic. Don’t let the word “summary” fool you. Depending on the product and complexity, this module can easily run 40-80 pages in length, not inclusive of diagrams and tables.
eCTD Module 3: Quality Related Information (Pharmaceutical Documentation)
Module 3. of an eCTD submission focuses on manufacturing and has sections covering the Drug Substance and the Drug Product (manufacturing, pharma development, product/ excipient control, etc). Because it is important to understand the differences between the active substance and the final drug product, the Substance and Product sections share many common subsections focused on manufacturing, substance/product control, stability, manufacturing, and container closure systems.
Unlock and explore the true value of your regulatory dossiers, NeeS & other regulatory dossier formats in a safe and secure, web-based eCTD viewer.
eCTD Module 4: Non-clinical Study Reports (Pharmacology and Toxicology)
The results of your non-clinical studies will be presented in module 4. It is here that you will upload all individual study reports and list proper literature references. There are major subsections for pharmacokinetics, pharmacodynamics, carcinogenicity and toxicology. The overviews in this section typically should not be more than 30 pages but the written summaries are often 100-150 pages.
eCTD Module 5: Clinical Study Reports (Clinical Trials)
All clinical study reports, raw data, and associated information go here. This is where you will add a critical analysis of your clinical data, a rationale for product development and overviews of biopharmaceutics, clinical pharmacology, efficacy and safety. Literature references will also be included along with various clinical summaries. Clinical Study Reports (CSR) in this module are organized by objective and are referenced in other modules.
The focus here is on presenting the data to support the safety and efficacy of the drug or biologic along with the benefit-risk analysis.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
eCTD Guidelines and Templates
ICH publishes a wide variety of guidelines and templates here but you should reference eCTD guidance documents published by the US FDA, Europe, or the market regulator to which you will be submitting your application.




