
IDMP Compliance with Effective Master Data Management
Master data management is crucial for ensuring data integrity and consistency. This is especially important in highly regulated industries like pharmaceuticals, where accurate information is critical. By establishing a single source of truth for master data, organizations can reduce errors, improve compliance, and make better decisions. Ultimately, effective master data management can lead to safer and more effective healthcare outcomes for patients. All stakeholders benefit from reliable and consistent information when it comes to pharmaceuticals.
In today’s data-driven world, the integrity and consistency of information are paramount, particularly in highly regulated industries such as pharmaceuticals. Master Data Management (MDM) emerges as a crucial framework for ensuring that organizations maintain accurate and reliable data. In this blog, we will explore the significance of MDM, its benefits, and how it contributes to better healthcare outcomes.
Understanding Master Data Management
At its core, Master Data Management refers to the processes, governance, and tools that organizations use to create and maintain a single source of truth for their critical data. This includes data about products, customers, suppliers, and more. In the pharmaceutical sector, where compliance with regulations is essential, MDM becomes even more vital.
Enhancing IDMP Preparation through Master Data Management
- MDM Overview: Master Data Management (MDM) is a comprehensive method for ensuring the accuracy, consistency, and accountability of an organization’s shared data assets.
- IDMP Overview: The Identification of Medicinal Products (IDMP) is a set of standards developed by the International Organization for Standardization (ISO) to improve the identification and data exchange of medicinal products globally.
By leveraging MDM systems and processes, life sciences companies can streamline their IDMP preparation efforts, ensuring they meet regulatory requirements efficiently and effectively.
Key IDMP requirements include:
- Product Identification: Unique identifiers for medicinal products.
- Data Attributes: Specific data elements like product name, dosage form, strength, and packaging.
- Regulatory Compliance: Adherence to global regulations for product data reporting.
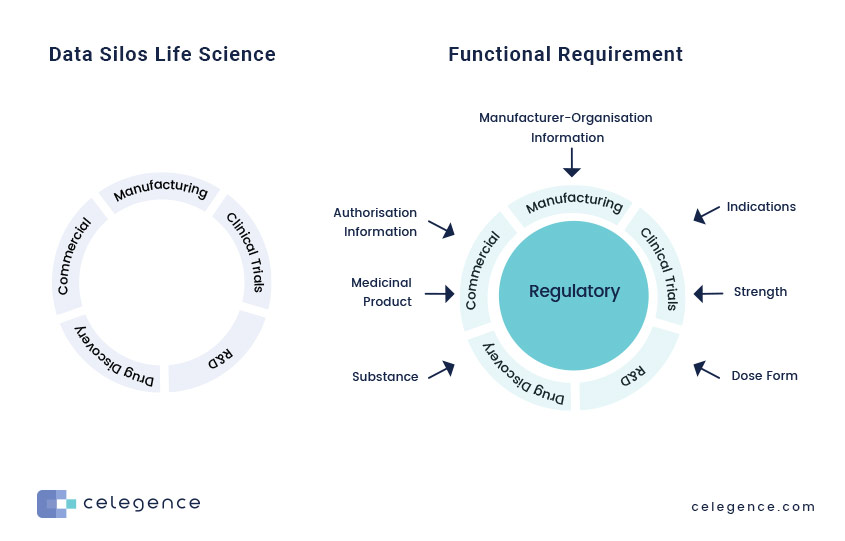
Opportunity for Consolidation across Drug Development Value Chain
IDMP compliance as an opportunity to:
- Build a repository of enterprise-wide Data Assets
- Identify opportunities for and improve Data Quality and Data Governance practices
- Create an Enterprise Architecture view for data across the Drug Development Value Chain
- Improve Operational Efficiencies
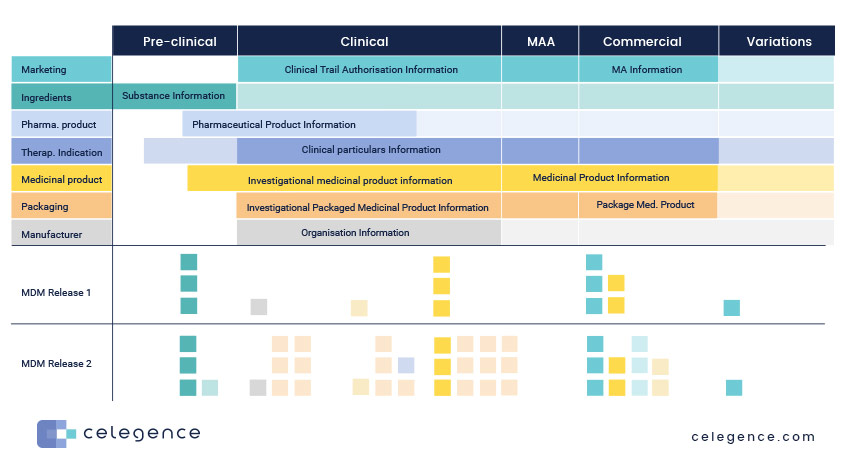
IDMP necessitates that input from multiple functions and systems within the organization are brought together to stay consistently compliant, as outlined below:
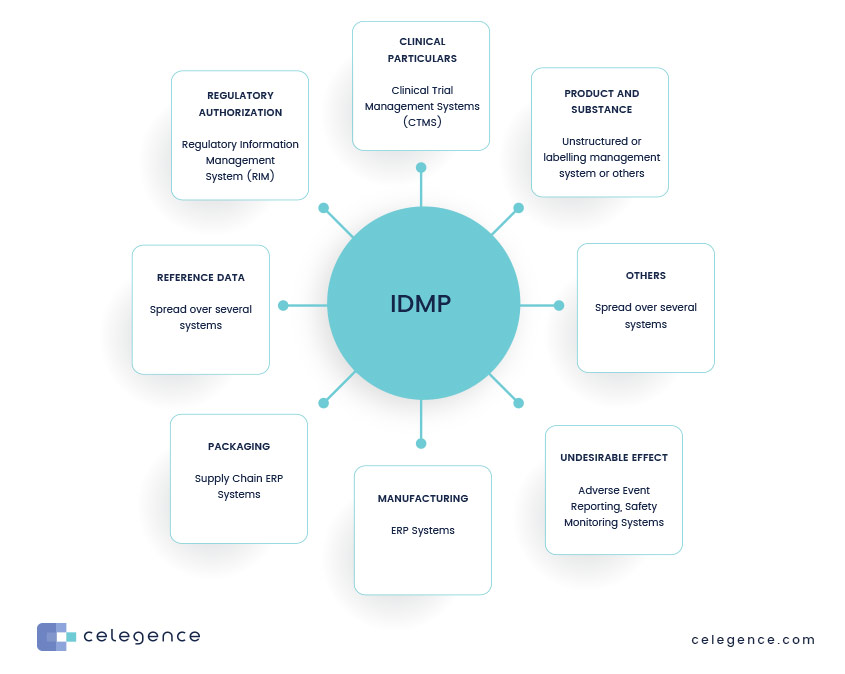
Master Data Management (MDM) for IDMP
Master Data Management (MDM) systems and processes can significantly enhance IDMP (Identification of Medicinal Products) preparation in several ways:
- Data Standardization: MDM helps standardize data formats and terminologies across the organization, ensuring consistency in product information. This is crucial for compliance with IDMP standards, which require specific data attributes.
- Centralized Data Repository: MDM provides a centralized repository for all product-related data. This enables easy access and management of information, reducing data silos and ensuring that all stakeholders have access to the most up-to-date information.
- Data Quality Improvement: MDM processes include data cleansing and validation, which enhance the accuracy and reliability of product data. High-quality data is essential for meeting IDMP requirements.
- Unique Identification: MDM systems facilitate the assignment of unique identifiers to medicinal products, a key requirement of IDMP. This ensures that each product can be distinctly recognized throughout its lifecycle.
- Regulatory Compliance: MDM systems can be designed to align with regulatory requirements, making it easier to track compliance and generate necessary reports for IDMP submissions.
- Integration Across Systems: MDM enables integration between various systems (like R&D, manufacturing, and commercial) to ensure that product data is consistent and up to date across all departments.
- Enhanced Reporting and Analytics: MDM systems provide tools for reporting and analytics, helping organizations monitor compliance status and identify gaps in data that need to be addressed for IDMP readiness.
- Collaboration and Workflow Management: MDM processes promote collaboration among different teams (e.g., regulatory affairs, quality assurance, and R&D) by establishing clear workflows for data management and approval processes related to IDMP.
- Change Management: MDM systems can help manage changes in product data efficiently, ensuring that updates are reflected across all relevant systems and that historical data is maintained for compliance purposes.
- Training and Awareness: Implementing MDM processes can also involve training staff on IDMP requirements and the importance of data management, fostering a culture of compliance within the organization.
Regulatory Support and Technology Solutions to Life Sciences Companies
At Celegence, we specialize in providing comprehensive regulatory support and technology solutions to life sciences companies. Our expertise in Master Data Management (MDM) helps organizations establish a single source of truth, ensuring data consistency, accuracy, and regulatory compliance. Leveraging our deep industry knowledge and innovative tools, including our Dossplorer™ platform, we support clients to streamline their IDMP preparation, enhance data governance practices, and achieve operational efficiencies. With Celegence as your partner, you can confidently navigate complex compliance requirements and focus on delivering safer and more effective healthcare solutions to patients.
Connect with Celegence, led by Sonia Veluchamy, to address any IDMP regulatory concerns and discover how our team of experienced IDMP regulatory affairs consultants can assist you in achieving successful regulatory outcomes for your product.

IDMP Compliance Specialists
Seamlessly ensure IDMP compliance with global regulatory experts and groundbreaking software from Celegence. Discover how our IDMP services can revolutionize your regulatory operations for efficiency, accuracy, and compliance. Reach out to us at info@celegence.com to learn more.
About The Author

Sowmya Raju is a Senior Regulatory Subject Matter Expert (SME) at Celegence, bringing extensive experience in xEVMPD, IDMP, RIMS, and Regulatory Affairs. She actively contributes as a member of the Regulatory Information Standards Subcommittee (RISS) Technical Group, focusing on IDMP, Computer System Validation (CSV), and providing validation and implementation support.


