
IDMP Compliance for Pharma: Strategy & Benefits
In the ever-evolving landscape of pharmaceuticals, the introduction of standards to uniquely identify medicinal products (IDMP) is pivotal. But what does this mean for pharma companies? Let us look into some real-world implications and why it is more than just another regulatory hurdle.
ISO IDMP and Compliance Requirements: Beyond Ticking Boxes
IDMP standards, encapsulated in ISO 11238, ISO 11239, ISO 11240, ISO 11615, and ISO 11616, are not just about ticking boxes. They represent a fundamental shift towards better data management allowing for interoperability. Achieving compliance is no small feat. Pharma companies must break down data silos and ensure seamless integration across departments. This is easier said than done, especially for large organizations. The challenge is real, but so are the rewards.
Compliance with IDMP standards requires a comprehensive approach to data governance. Companies need to establish robust processes for data collection, validation, and maintenance to ensure the accuracy and consistency of their product information. This involves not only updating existing data but also implementing systems to capture new data in a standardized format. The effort required to achieve this level of data management can be significant, but the benefits of having a single source of truth for product information are invaluable.
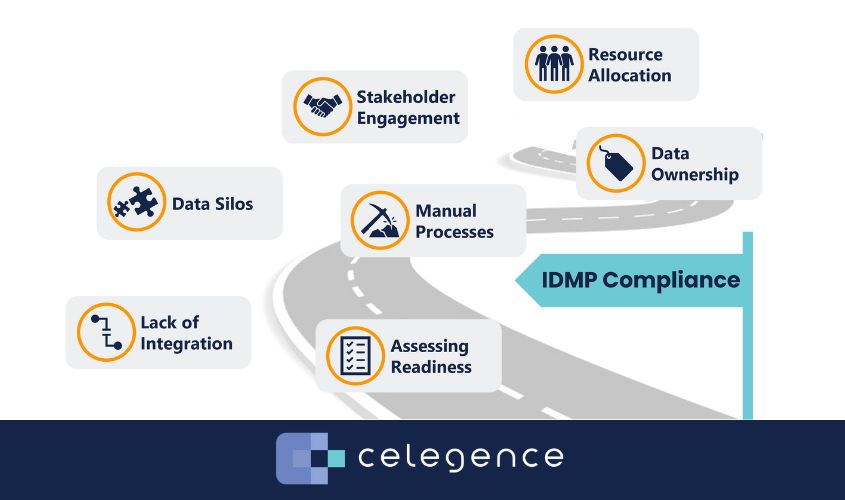
Key Pitfalls and Readiness: Navigating the Minefield
The road to IDMP compliance is fraught with pitfalls. Data silos, manual processes, and lack of integration are common stumbling blocks. Companies must also contend with issues of data ownership and resource allocation. Assessing readiness is crucial. Companies need to thoroughly investigate their current data practices and identify gaps. Smart investments in training and resources now will pay dividends down the line. The question is, are you ready to make that investment?
One of the biggest challenges in achieving IDMP compliance is breaking down data silos. In many organizations, data is stored in disparate systems that do not communicate with each other, making it difficult to get a complete and accurate picture of product information. To address this, companies need to invest in solutions that enable data integration and ensure that all relevant data is accessible from a single platform. This may involve significant changes to existing IT infrastructure and processes, but the long-term benefits of having integrated data systems are well worth the effort.
Another critical aspect of readiness is ensuring that all stakeholders are on board with the changes required for IDMP compliance. This includes not only regulatory teams but also other departments such as manufacturing, quality assurance, marketing and IT. Effective communication and collaboration across departments are essential to ensure that everyone understands the importance of IDMP and is committed to achieving compliance
Why IDMP is Being Introduced: The Bigger Picture
As a reminder, IDMP standards aim to enhance patient safety, improve regulatory compliance, and facilitate global collaboration. This is about creating a more transparent and efficient pharmaceutical ecosystem. For companies, it is an opportunity to streamline operations and gain a competitive edge. But it requires a shift in mindset – from viewing compliance as a burden to seeing it as a strategic advantage.
The primary goal of IDMP is to improve patient safety by ensuring that accurate and consistent information about medicinal products is available to healthcare professionals and regulatory authorities. This is particularly important in the context of global supply chains, where products may be manufactured, distributed, and used in different countries with varying regulatory requirements. By standardizing product information, IDMP helps to ensure that all stakeholders have access to the same data, reducing the risk of errors and improving patient outcomes. Additionally, IDMP plays a crucial role in addressing medicine shortages by providing a unified framework for monitoring supply and demand, as seen with the European Shortages Monitoring Platform.
IDMP also supports regulatory compliance by providing a standardized framework for submitting product information to regulatory authorities. This can help to streamline the approval process for new products and variations, reducing the time and cost associated with regulatory submissions. For companies, this means faster time to market and a competitive advantage in the global marketplace.
Benefits of IDMP and Digital Continuity: The Silver Lining
Despite the challenges, the benefits of IDMP are substantial. Better data quality and consistency lead to improved regulatory compliance and reduced risk of errors. Efficient data exchange with regulatory authorities can streamline the approval process for new products. In the long run, IDMP can drive innovation and growth, and the standards offer an opportunity for digital continuity. By standardizing data and improving interoperability, companies can achieve a seamless flow of information across the product lifecycle. This not only enhances regulatory compliance but also supports innovation and collaboration. Embracing digital continuity is key to staying competitive in a rapidly evolving industry.
By adopting IDMP standards, companies can ensure that their product information is always up-to-date and accessible, regardless of where it is stored or how it is used. This can help to improve decision-making, reduce the risk of errors, and support more efficient and effective operations. Furthermore, IDMP also provides a common framework for data exchange, making it easier for companies to work together on projects to drive innovation and accelerate the development of new treatments and therapies.

FHIR and IDMP-O: Enhancing Interoperability
Fast Healthcare Interoperability Resources (FHIR) is a critical component in the digital transformation of the pharmaceutical industry. It provides a standardized format for data exchange, enabling efficient storage and transfer of information. This is particularly important for IDMP compliance, as it ensures that data can be easily shared and accessed across different systems and organizations. By adopting FHIR, pharma companies can enhance their data interoperability, streamline regulatory submissions, and improve overall operational efficiency.
The IDMP-Ontology (IDMP-O) project, spearheaded by the Pistoia Alliance, aims to create a shared ontology to encourage uniform adoption of IDMP standards and consistent exchange of information. This initiative is crucial for enabling cross-functional data integration and supporting broader digitalization efforts within pharma companies. Interoperability is a key enabler of digital transformation in the pharmaceutical industry. By adopting FHIR and IDMP-O, companies can ensure that their data is compatible with other systems and can be easily shared and accessed by all stakeholders to support more efficient and effective operations.
Contact Celegence for Full Suite of IDMP Services
Navigating the complexities of IDMP compliance can be daunting. At Celegence, we offer comprehensive IDMP compliance consulting services to support pharmaceutical companies. Our team of regulatory experts can assist with organizational readiness assessments, data collection, maintenance, submission, and governance.
We provide both short-term and long-term strategic approaches to help you respond to regulatory requirements and establish broader benefits for your business. Contact us today at info@celegence.com to learn how we can help you achieve IDMP compliance and secure your pharma company’s ticket to the future.
About The Author

As a manager within Regulatory Data Management at Celegence, John has extensive experience in xEVMPD, IDMP, RIMS, and Regulatory Affairs. John has supported numerous pharmaceutical companies in their digital transformations by selecting the right regulatory information management system, extracting & remediating regulatory data, reviewing and updating underlying processes, and fostering a culture of collaboration and innovation among teams to drive successful change.


