Leveraging the Business-Process-as-a-Service (BPaaS) Model in Regulatory Affairs
The Life Sciences industry has not yet fully leveraged the Business-Process-as-a-Service model (BPaaS) for Regulatory Affairs operations, leaving ample opportunity to apply this model to achieve cost savings and efficiencies. Celegence offers its customers the BPaaS model for end-to-end Regulatory services by combining the best-in-class technology with efficient processes for end-to-end product lifecycle management. This includes regulatory strategy, content authoring, content management, publishing, RIMS data management, compliance data management, labeling, and cross-functional information exchange with sales, marketing, manufacturing, and logistics. When using cloud solutions, life sciences organizations can benefit by shifting the responsibility of application and platform maintenance to Celegence, thereby eliminating capital expenditure and time spent managing technology solutions and vendors.
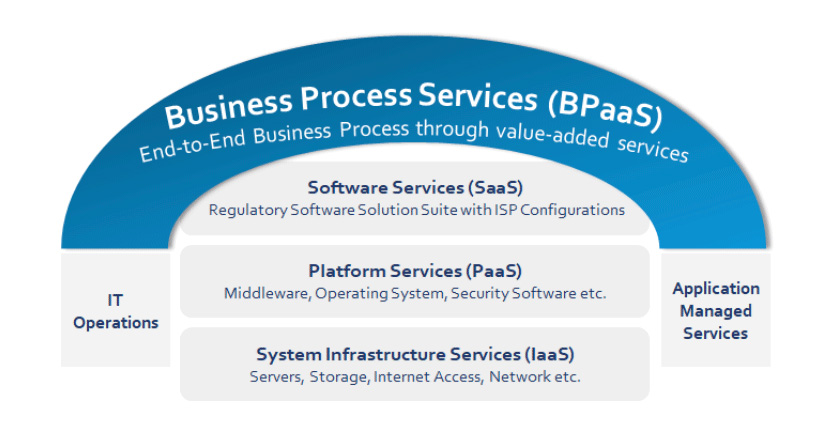
BPaaS Model for End-to-End Regulatory Services
This flexible delivery model is particularly beneficial to companies that do not have the infrastructure in place and want to increase market access. Such companies can avoid having to conduct extensive due diligence and investing heavily in new technologies. Celegence can manage all relevant activities required for rolling out a new system or process, including the data collection and data migration into the future solution platform. We work with clients to identify the business outcomes that matter and define performance and quality metrics that we can be measured against, thus providing them with greater transparency.
BPaaS Model for Maximum Efficiency
The model is not limited to small companies, as midsize and larger companies are also looking for ways to become more efficient, reduce cost and reduce the number of vendors while maintaining compliance. BPaaS allows for companies to work with a single partner to maximize the efficiency in their operations. In this light, Celegence will own the business processes and the platform in whichever operational area the customer might choose: be it publishing, labeling, or RIMS data management. We can manage these operations for your medicinal products that may be in any stage – from R&D to divestment.
On a day-to-day basis, the only piece of the process that will be visible to you and your team will be the business layer, allowing you to focus on mission critical activities. We ensure that our process-driven services are done with a blended resource model composed of highly trained experts.
Finally, BPaaS allows life sciences companies to achieve greater transparency and stronger reporting so that they can actively monitor productivity. When leveraging this model, companies can shift to a transaction-based payment with a single vendor for Regulatory Affairs operations, which will allow them to easily measure performance and quality.


