Effective Post-Market Surveillance for Medical Devices
Post-market surveillance (PMS) is defined as “a systematic process to derive necessary corrective and preventive actions (CAPA) from information on medical devices that are already placed on the market”. The activities are designed to identify problems in the design of the device or accurately point to device failures, aimed at ultimately improving clinical outcomes.
The following is the eleventh in the series of EU MDR related blogs. To learn more about the EU MDR changes you can view some of the previous posts in the series:
- Medical Device Equivalence vs Demonstration of Equivalence
- Post-market Clinical Follow-up Requirements for EU MDR
- The New European Union MDR: Impact on Technical Files
- Low-risk Medical Device Challenges
- Selecting and Working with your Notified Body
- Medical Devices with Ancillary Medicinal Substances
- Regulatory Challenges Writing EU MDR Compliant Clinical Evaluation Reports (CER)
- International Medical Device Regulators Forum (IMDRF) & Summary of Recent Changes to Clinical Evaluation Guidance
- Remote Medical Audits During COVID-19
- Celegence Webinar: Taking advantage of the EU MDR Delay in Uncertain Times

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
PMS Requirements – MDD Compared to MDR
Previously, PMS requirements were considered synonymous with manufacturer responsibilities in post-production phase as EU Medical Device Directives (MDD) PMS requirements were the same as the post-production monitoring requirement of ISO 14971 clause 9. The lack of clarity has now been addressed with MDR, where PMS has been defined in Article 2 (60) as one of the general obligations of all manufacturers. Additionally, MDR has specifically called out for personnel responsible for monitoring ensuring regulatory compliance.
Manufacturers are now required to update their PMS system proactively in a comprehensive and systematic fashion in accordance with EU MDR. This allows the manufacturers to set in place an interactive corrective action or preventive action process which is proportionate to the device type and update clinical evaluation documents.
It is important to note that the requirements for PMS should be directly proportional to the risk associated with the device based on its intended use.
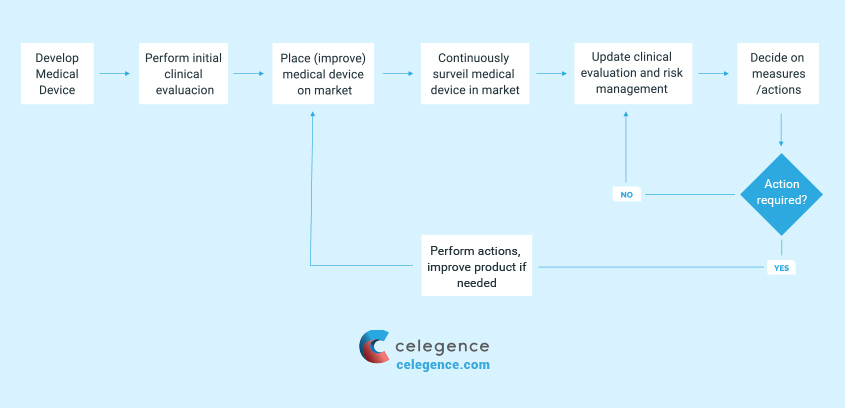
Requirements of Post Market Surveillance (PMS)
It is necessary to consider the product technology in relation to the manufacturer and the marketplace while developing a PMS, as there tends to be a large amount of data informing about the reasonable risks associated with the device when the device technology has been in place over a prolonged period of time. For manufacturers of new technology, there may be limited understanding of the patient population and complexities of the device state. Requirements of PMS can be met by:
- State-of-the-art market experience data for similar devices may be adequate to support clinical evaluation requirements for low risk devices with a long history of use
- Quantified clinical data pertaining to safety and performance of the device from peer-reviewed literature in order to support the clinical evaluation requirements
- Increased monitoring ensuring early detection of risks which were not foreseen in the development phase
- Post-market clinical follow-up (PMCF) may also be warranted to capture adequate real-world clinical use of the device
Manufacturers must document and demonstrate conformity to the ‘General Safety and Performance Requirements (SPRs) which must be updated in response to PMS activities.
PMS As Risk Management
The analysis and review of PMS data is to be performed regularly by manufacturers as part of the risk management process. Information for risk management can be gathered from patients, physicians, healthcare facilities, regulatory authorities, professional societies, researchers and internal personnel.
A combination of ‘proactive’ and ‘reactive’ PMS activities form the basis of the device’s PMS plan, which is provided as a part of the assessment for CE mark certification. Proactive PMS anticipates and curtails events before they occur, gaining real-time performance data of the device. Examples include, customer surveys, post CE mark clinical trials (including PMCF), expert user groups, and manufacturer sponsored device tracking/implant registries. Reactive PMS is a passive form of data collection activities when manufacturers respond after an event has occurred. It could range from complaints to serious injury. Examples include, customer complaints, service reports, failure analysis, literature review, and non-manufacturer sponsored trials or regional/national device registries.
Quality Management System
Based on the classification of a device, a quality management system (QMS) should cover all procedures and processes relating to aspects of design, manufacturing, supply management, risk management, complaint handling, clinical data, storage, distribution, and product labeling.
Manufacturers of all medical devices must document and prove to the notified bodies that they meet the requirements of Article 10 (General obligations of manufacturers) and Annex IX (Conformity assessment based on QMS and technical documentation) of the new EU Medical Device Regulations (EU MDR). Additional ways to meet QMS requirements include compliance with the requirements enlisted in Annexes X (Conformity assessment based on type examination) and XI (Conformity assessment based on product conformity verification).
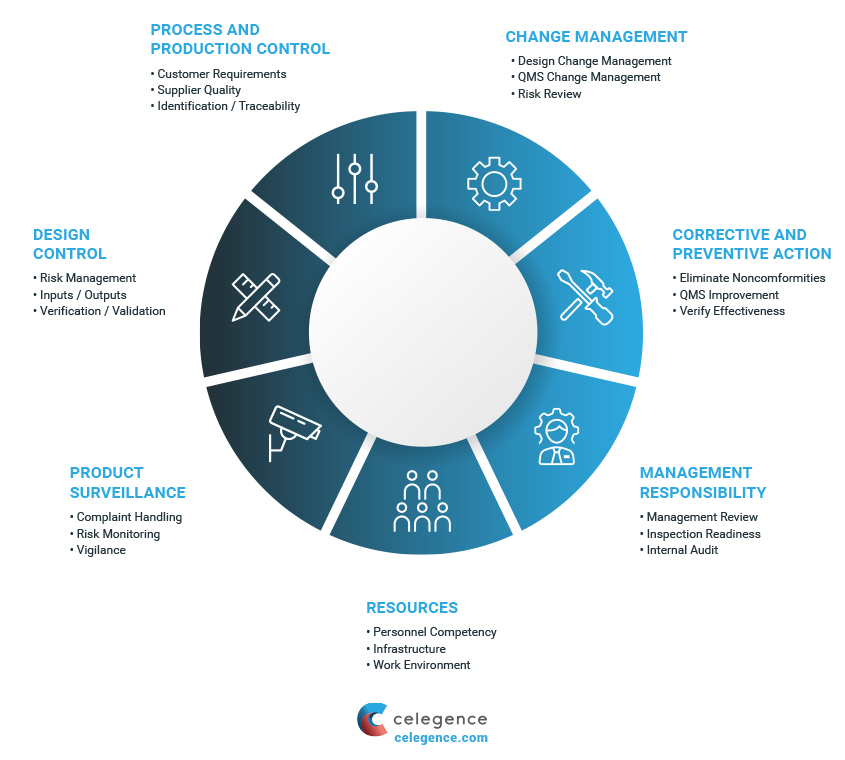
Pre-Production QMS
It is considered best practice to setup a pre-production QMS, 18-24 months prior to commercialization and distribution of the device. This helps manufacturers continuously update and improve the system while they proceed internal validation procedures and market launch. In order to ensure ongoing compliance with quality system procedures, manufacturers must be encouraged to perform internal audits evaluating QMS at least once a year.
Vigilance System in PMS
ISO 13485 applies to all medical devices on the market and therefore PMS plans must be formulated in context to a proactive PMS.
Vigilance is an output of the quality management system and is therefore beneficial to plan post-market clinical data gathering activities during the technical documentation of a device.
Therefore, establishing a vigilance system in the post-production phase to report authorities of serious device-related incidents (reactive in nature) could effectively facilitate the detection of previously unknown adverse product information. This assessment can prove useful to prevent future recurrence of incidents that might have otherwise led to death or serious deterioration in health.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
Vigilance Guidance Document
The Medical Device Vigilance System (MDVS) is intended to facilitate a direct, timely, and harmonized implementation of Field Safety Corrective Actions (FSCA) and FSN (Field Safety Notice) across the Member States where the device is in use.
The latest update in MDVS guidelines introduces manufacturer incident report (MIR) which details adverse event terminology/coding harmonized via the International Medical Device Regulators Forum (IMDRF) and the use of unique device identification (UDI), while introducing a single registration number (SRN), keeping in focus the future Eudamed.
Notified bodies provide support by assessment of vigilance procedures, performing audit procedures, and assessing the impact of vigilance issues on certification. Additionally, user input and feedback is vital for vigilance. Users report suspecting incidents with user interaction and cooperation is necessary for the implementation of FSCA.
Translating Clinical Data Beyond PMS Activities
Clinical data can help in identifying residual risks of devices. Additionally, gathering robust long-term clinical data can present itself as a successful marketing tool improving economic value of the device and ultimately support product liability claims, if required. The current emphasis on PMCF requirements by EU MDR in obtaining continued clinical data stands to benefit both patients and manufacturers. A PMCF study or plan is expected to be a part of the PMS plan and in the clinical evaluation. Adequate rationale is to be provided as to why a PMCF is not applicable for a medical device.

Effective Post-Market Surveillance for Medical Devices – Conclusions
A robust PMS program must provide, broad real-world experience beyond the confines of a re-market trial, detect early problems and ensure corrective actions in compliance with relevant legislations, and continue to monitor the long-term performance of the device adding value beyond compliance. This can be achieved by setting a competent QMS system in place that keeps track of a products life cycle, an adequate PMS plan, and continued clinical follow up.


