Celegence adds report writing functionality to EU MDR and IVDR compliance technology, CAPTIS™
Chicago, July 6th, 2022 – Celegence, a global provider of regulatory affairs services and solutions for the life sciences industry, has today announced the addition of new features to CAPTIS™ to help the device and diagnostic industry produce compliant Post Market Surveillance (PMS) documentation more efficiently.
Manage Systematic Literature Reviews Efficiently
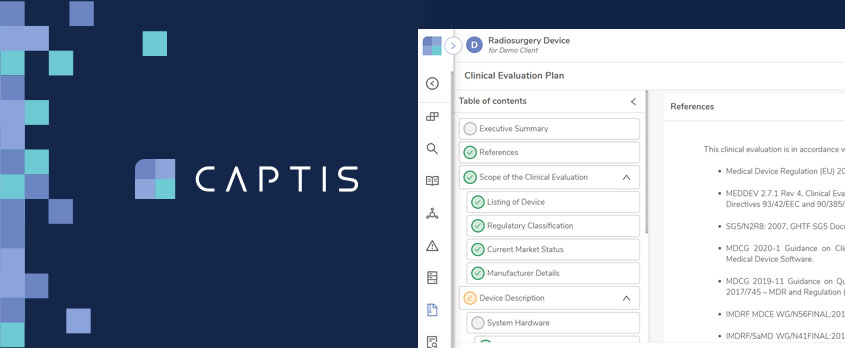
To comply with the EU Medical Device Regulation (MDR) and In-Vitro Diagnostic Regulation (IVDR), device and diagnostic manufacturers are required to produce and maintain various documentation such as Clinical Evaluation Reports (CER) and Post Market Safety Reports. Writing these reports is a complex and laborious process and CAPTIS™, a secure, user-friendly cloud solution, now features a report writing module designed to help medical writers manage systematic literature reviews more efficiently, streamline document lifecycle management, and facilitate the first-time acceptance of regulatory documentation by Notified Bodies.

Claim Your Free EU MDR Checklist Now!
Make sure you and your business are compliant with the new EU MDR. Get our 23 page checklist for actionable technical documentation requirements.
EU MDR & IVDR Report Automation
Medical writing teams typically must write and maintain a significant amount of documentation for a single device. CAPTIS™ automates this process, saving many hours of manual effort as well as significantly reducing the propensity for human error. By integrating seamlessly with databases such as PubMed and Google Scholar, it streamlines the screening and assessment of literature. A master data dictionary also saves time by allowing relevant information to be used consistently across various reports for the same device or device family.

CAPTIS™ Background
Celegence started building CAPTIS™ in 2019 as it foresaw the compliance challenges medical device manufacturers would face, since the company also provides consultancy and regulatory services to the devices and diagnostics industry. Manufacturers can use this web-based solution through their own medical writing team or in collaboration with Celegence’s medical writers as an extension of their team.

CAPTIS™ Goals
Punya Abbhi, COO at Celegence said: “As service providers to the industry, we appreciate the operational demands of preparing complex, compliant medical device reports as we face them as well. We have invested in the research and development of the CAPTIS™ solution to specifically address the challenges faced by device manufacturers.”
“Our goal with CAPTIS™ is to improve outcomes for the life sciences industry. We recognized the need to drive process efficiencies in the development of these reports and ultimately help medical device and diagnostic manufacturers achieve and maintain MDR & IVDR compliance.
“In addition to bringing efficiencies to medical writing and document maintenance, CAPTIS™ also helps improve the review process for Notified Bodies. As medical writing professionals, we work closely with Notified Bodies and recognize the pressures they face when it comes to reviewing huge volumes of information and references for each submission. Submitting these documents using CAPTIS™ ensures they are easily accessible and presented in compliant templates that are Notified Body approved.”

Experience CAPTIS™
Device and diagnostic manufacturers are invited by Celegence to experience CAPTIS™ for themselves. To arrange a demo, please contact a Celegence representative today at info@celegence.com or schedule your demo online.

