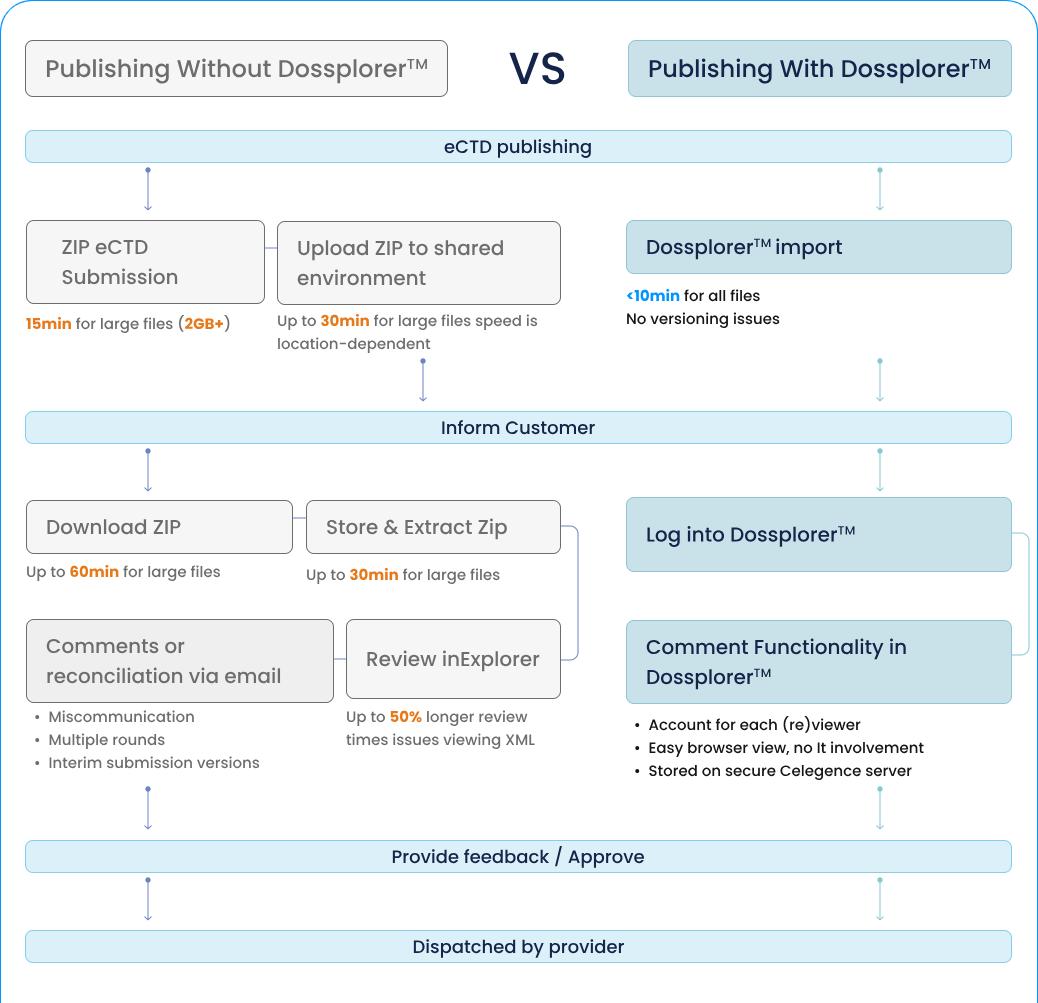
Creating well-written and structured documents is crucial for drug approval and submission. Regulatory medical writing involves compiling information from clinical trials, safety reports, and other key documents to produce submission-ready materials for M2, M4, and M5 modules.
Celegence’s global medical writing specialists are experts in drug development, multiple therapeutic areas and product types. We have in-depth knowledge of regulatory, clinical, and scientific content, integrated with technical, editorial, and quality control skills. Our writers collaborate with various stakeholders, including clinical operations, data management, biostatistics, medical, and safety teams. They bring extensive industry experience from pharmaceuticals, medical devices, CROs, service providers, and academia, covering all forms of medical and scientific writing.