Dossplorer™ allows you to share, view and review eCTD, NeeS and other dossier formats from any region and access them from any location. Unlock and explore the true value of your regulatory dossiers in a safe and secure, web-based eCTD viewer.
The hybrid cloud/ on-site solution offers you cloud-based software as a service whilst keeping your data privately stored on-site or in a virtual private cloud. Alternatively, Dossplorer™ can be installed as a full on-site solution.
Unlock and explore the true value of your regulatory dossiers in a safe and secure, web-based eCTD viewer.
Dossplorer™ At A Glance
Import Dossiers in Dossplorer™ eCTD Viewer
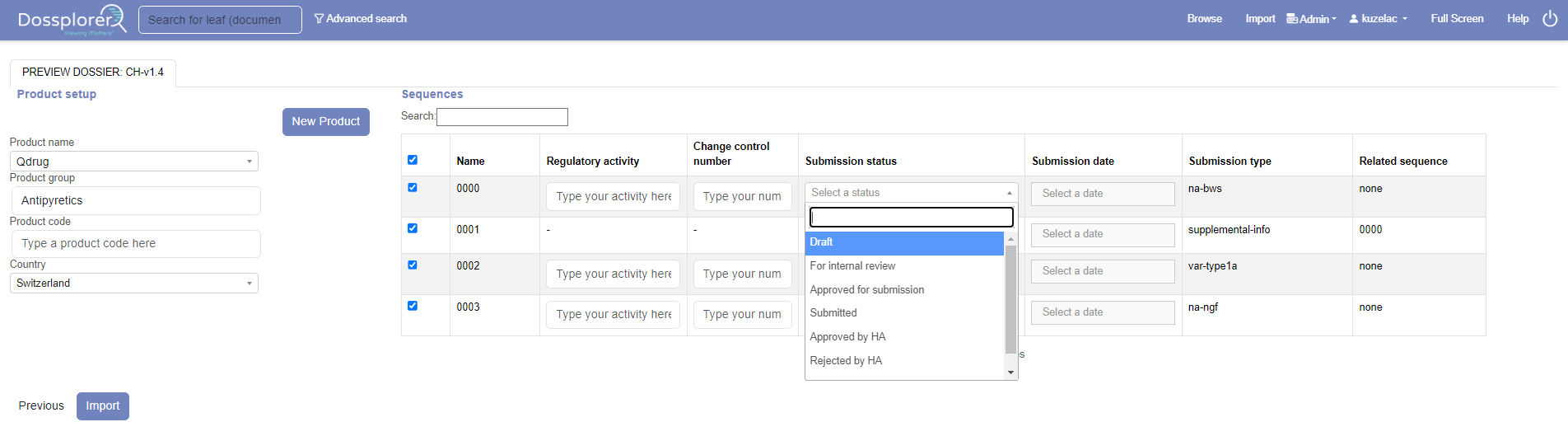
Explore Across Dossiers
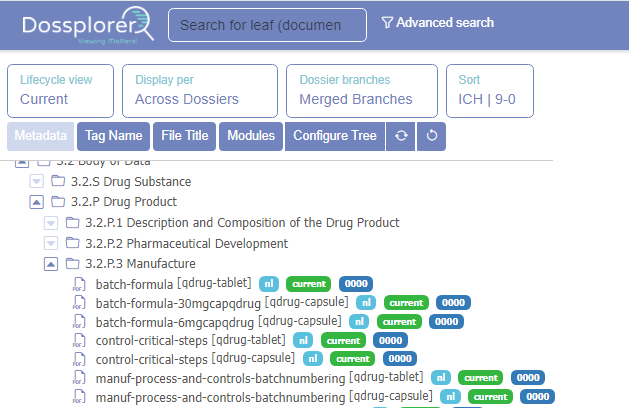
Personalized Navigation Tree
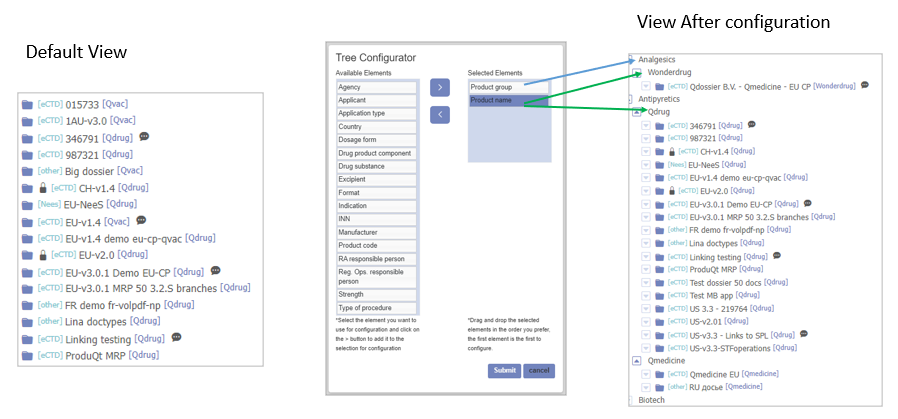
Review, Add Comments to eCTD, NeeS & Other Dossiers
Key Features of Dossplorer™ eCTD viewer
Import Regulatory Dossiers
Dossplorer™ functions as your own safe repository to import full application and individual submissions. Dossiers are automatically enriched with metadata extracted from the submission, which unlocks the true value of your information assets. Optionally, additional metadata can be added manually as part of the import process.
Explore Across Dossiers & Products
Dossplorer™ provides a holistic view across products and applications worldwide. As a result, users can create a consolidated view across dossiers in a single CTD structure. This allows for easy comparison of content and data present across multiple eCTDs.
Personalized Navigation Tree
You can personalize the navigation tree utilizing metadata already present in your dossiers. For example, the tree configurator allows you to change the hierarchical organization of dossiers. Moreover, the view settings, expanded nodes and selected metadata are stored and available after re-login.
Multiple Dossier Formats Supported
Dossplorer™ is an eCTD viewer, NeeS viewer and supports viewing any file and folder structure. Therefore, you can import virtually any regulatory dossier, including medical device dossiers, CTA dossiers, Paediatric Investigation Plans (PIP) and Investigational Medicinal Product Dossiers (IMPD).
Dossier Review
You can review the dossier and add the comments to eCTD, NeeS and other dossiers. Likewise, Dossplorer™ can be used to support basic internal review workflows, eCTD life cycle management and submission publishing.
Unique Views on eCTDs
Dossplorer™ facilitates creation of current and cumulative views on individual regulatory activities and merge branches (e.g. 3.2.P branches for drug products or 3.2.S branches for drug substances) into a single branch. As a result, you can easily compare documents across different strengths, dosage forms and manufacturers.
Advanced Restrictions
Advanced node restrictions can be applied to sequences or sections within a dossier in order to limit the access. This feature allows you to restrict specific contents such as non-blinded clinical study data or confidential details about quality and manufacturing to a limited group of users.
Automated Import
Dossplorer™ Xchange Bot scans for new dossiers and sequences published on the connected network locations. Consequently, the import agent detects the submissions and import them automatically. Dossplorer™ only stores references to the files and does not duplicate the physical content files.
Hybrid-cloud, Full Cloud or On-Site Architecture
The hybrid cloud / on-site solution offers you cloud-based software as a service whilst keeping your data privately stored on-site or in a virtual private cloud. Alternatively, Dossplorer™ can be installed as a full on-site solution. User only needs a web browser such as Chrome or Edge to connect, without any local installation required.
eCTD Viewer with Single Sign-on Solutions
Safe and secure access to Dossplorer™ can be facilitated with single sign-on applications, such as Okta, PingFederate, Microsoft 365.
Are You Looking for Personalized demonstration of Dossplorer™ eCTD viewer?
Learn how your business can increase efficiencies in preparing eCTD dossiers with Dossplorer™ and fill in the below to contact us today.

