
Partnership Program
Become a trusted CAPTIS™ partner and you will have access to the full suite of features and capabilities to enhance your documentation processes.
Through the CAPTIS™ Partnership Program, medical writing teams can leverage Celegence’s productivity enhancing technology.
With various licensing agreements available, service providers can select the option that best fits their needs.
For a deep dive into CAPTIS™, or to download the CAPTIS™ brochure, you can explore our dedicated page here.
Since the CAPTIS™ solution was released for external use in 2021, numerous medical device teams have realized immense improvements in their CER and Literature Search Review processes. Integrations with medical research databases such as PubMed and Google Scholar with automatic citation data and full-text retrieval minimizes the manual aspects.
Additional integrations with major adverse event databases such as US FDA MAUDE and TPLC allows the end-user to complete the process without leaving the CAPTIS™ platform.
CAPTIS™ Benefits
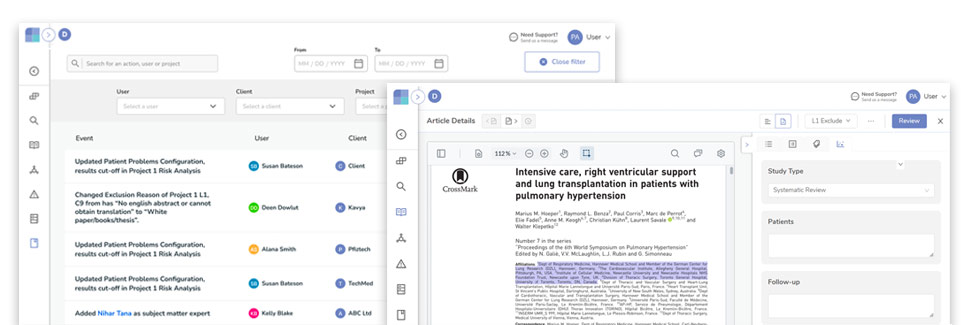
By becoming a trusted CAPTIS™ partner, you will have access to the full suite of features and capabilities to enhance your documentation processes.
Meet CAPTIS™ – Your New EU MDR Compliance Tool
Testimonials
“Literature search results are far more reproducible and auditable with CAPTIS™.”
“A lot of my time is saved by utilizing one platform to access multiple databases.”
“CAPTIS™ is a very innovative and time saving platform and makes the CER writing process quick and convenient.”
“A majority of our manual processes are now automated with the help of CAPTIS™.”
CAPTIS™ News
Redefining Compliance for EU MDR & IVDR
Maximize the Efficiency of your Medical Writing Team with CAPTIS™
To view a demo of the platform, or to speak with a Celegence representative regarding how your team could benefit from our CAPTIS™ partnership program, we encourage you to fill out the form below.




